THP1-KO-ASC Cells
-
Cat.code:
thp-koascz
- Documents
ABOUT
ASC knockout in human monocytes for inflammasome studies
THP1-KO-ASC cells are designed to foster research on the ASC adaptor during inflammasome formation. These cells feature a knockout (KO) of the human ASC (apoptosis-associated speck-like protein containing a CARD domain, also known as PYCARD) gene; thus, they have no functional ASC protein expression. THP-1 monocytes are widely used for inflammasome studies due to their high expression levels of NLRP3, ASC, and pro-caspase 1.
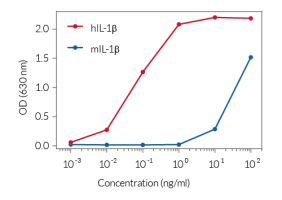
In THP1-KO-ASC cells, mature IL-1β secretion is abolished following stimulation with NLRP3 inflammasome inducers (e.g., Nigericin, MSU crystals, Alum), compared to their parental cell line THP1-Null2. Furthermore, NLRP3-driven pyroptosis is absent in ASC-KO cells, as confirmed by the LDH-Blue™ cytotoxicity assay (see figures).
THP1-KO-ASC cells are a valuable tool for investigating the role of the ASC adaptor protein in inflammasome responses. They can also function as a control cell line for the screening of novel therapeutics that target the ASC signaling pathways.
Key features
- Verified biallelic KO of the ASC gene
- Complete abrogation of mature IL-1β secretion
- Convenient readout using IL-1β HEK 293 reporter cells
- Stability guaranteed for 20 passages
ASC (apoptosis-associated speck-like protein containing a CARD domain, also known as PYCARD) is a protein adaptor important in canonical inflammasome responses [1]. ASC's bipartite composition, consisting of one PYD and one CARD domain, allows the recruitment of the CARD-containing pro-caspase-1 to canonical inflammasome sensors that do not contain a CARD domain, such as NLRP3, AIM2, and Pyrin [1].
To detect and quantify mature human IL-1β release, InvivoGen provides HEK-Blue™ IL-1β sensor cells, engineered to express an NF-κB-inducible SEAP reporter gene. SEAP activity can be rapidly measured using QUANTI-Blue™ Solution through the colorimetric readout at 650 nm. To visualize inflammasome formation using a microscope, InvivoGen also provides THP1-ASC-GFP reporter cells.
Moreover, for detecting and quantifying pyroptotic cell death, InvivoGen provides THP1-HMGB1-Lucia™ cells, which express a cytoplasmic HMGB1::Lucia luciferase fusion protein that is released in the supernatant upon pyroptosis. The activity of the Lucia luciferase reporter protein can be readily assessed using the QUANTI-Luc™ detection reagent.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
ASC
Human
Cellular assay for inflammasome activation
Complete RPMI 1640 (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:THP1-KO-ASC Cells
-
Cat code:thp-koascz
-
Quantity:3-7 x 10^6 cells
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Inflammasomes are multimeric protein complexes that are crucial for host defense against infection and response to endogenous danger signals. The canonical inflammasome response is driven by aggregation of a sensor (i.e. NLRP3) with the ASC adaptor and pro-caspase-1. Activation of caspase-1 (CASP1) induces the maturation of pro-IL-1β/pro-IL-18 and cleavage of the pore-forming protein gasdermin D (GSDMD), leading to secretion of IL-1β/ 18 and pyroptosis.
ASC is essential to canonical inflammasome sensors that do not contain a CARD domain, such as NLRP3, AIM2, and Pyrin [1]. ASC's bipartite composition, consisting of one PYD and one CARD domain, allows the recruitment of the CARD-containing pro-caspase-1 to these sensors. The NLRP1 and NLRC4 inflammasome sensors have a CARD domain, and can thus recruit pro-caspase-1 either directly, or through ASC. However, NLRP1 or NLRC4 activation in the absence of ASC triggers a reduced secretion of mature IL-1β and IL-18 [1]. Importantly, non-canonical inflammasomes (i.e. CASP4/5/11) activate CASP1 indirectly: their activation triggers GSDMD-driven release of alarmins and K+ efflux, which in turn, induce NLRP3- and CASP1-mediated IL-1β/-18 maturation and secretion. Therefore, the absence of ASC affects the downstream inflammatory signaling from the non-canonical inflammasome also.
In resting cells, ASC is present in a soluble and diffuse form both in the cytoplasm and nucleus [2]. Inflammasome activation in most cells leads to the formation of one large, micrometer-sized, ASC ‘speck’ per cell, thus concentrating CASP1 activation sites [2,3]. Yet, the number of ASC specks may dependent on the inflammasome inducer used. For example, Nigericine promotes the formation of numerous specks, whereas ATP leads to the accumulation of fewer specks [4].
1. Mathur A. et al., 2017. Molecular mechanisms of inflammasome signaling. J. Leuk. Biol. 103:233.
2. Hoss F. et al., 2017. Assembly and regulation of ASC specks. Cell. Mol. Life Sci. 74:1211.
3. Stutz A. et al., 2013. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol.
4. Zha Q. et al., 2016. ATP-induced inflammasome activation and pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front Immunol. 7:597.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?