Anti-hIL1RAP-hIgG1
-
Cat.code:
hil1rap-mab1
- Documents
ABOUT
Anti-human IL-1R3 (IL1RAP) - Nidanilimab biosimilar - CAS #2171061-85-9
Anti-hIL1RAP-hIgG1is a biosimilar antibody of Nidanilimab, a human interleukin 1 receptor accessory protein (IL1RAP or IL1R3) antibody that blocks IL-1α and IL-1β signaling. This monoclonal antibody (mAb), also known as Nadunolimab or CAN04, is under investigation for the treatment of various solid cancer types, including pancreatic cancer and refractory non-small-cell lung cancer (NSCLC).
Anti-hIL1RAP-hIgG1 comprises the variable region of Nidanilimab and the IgG1 constant region of Nidanilimab for high effector functions.
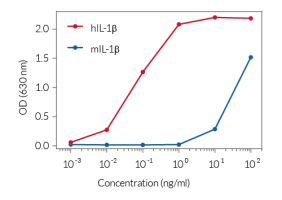
This mAb can be used together with HEK-Blue™ IL-1β cells for screening and neutralization assays to block IL-1 signaling induced by recombinant human IL-1β (see figure). Nidanilimab also inhibits the signaling of human IL-1α, IL-33, and IL-36α (data not shown).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
Human IL-1R3, IL-1RAP, IL1RAP, IL-1RAcP
Human
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Neutralization assay, ELISA
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL1RAP-hIgG1
-
Cat code:hil1rap-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Nidanilimab/Nadunolimab background
Nidanilimab (CAN04, Nadunolimab) is a fully humanized monoclonal antibody (mAb) that targets interleukin-1 receptor accessory protein IL-1R3, also known as IL-1RAP or IL-1RAcP [1].
IL-1RAP is a co-receptor essential for signaling by several IL-1 family cytokines, including IL-1α, IL-1β, IL-33, and IL-36 [1]. IL-1RAP mediates signal transduction by associating with the primary ligand-binding receptors IL-1R1, ST2, or IL-1R6. This activates the transcription factorsNF-κB and AP-1, leading to the expression of pro-inflammatory genes [1].
Nidanilimab was designed to disrupt the IL-1RAP-dependent signaling pathways and to induce antibody-dependent cellular cytotoxicity (ADCC) in IL-1RAP-expressing cells. As IL-1RAP is found to be overexpressed in multiple hematological and solid cancers, it is considered a promising therapeutic target. Multiple clinical trials were launched to assess the safety, tolerability, and efficacy of Nidanilimab, both as a monotherapy and in combination with chemotherapy regimens [1-3].
References:
1. Fields JK, et al., 2021. Molecular Basis of Selective Cytokine Signaling Inhibition by Antibodies Targeting a Shared Receptor. Front Immunol. ;12:779100.
2. Robbrecht D, et al., 2022. First-in-human phase 1 dose-escalation study of CAN04, a first-in-class interleukin-1 receptor accessory protein (IL1RAP) antibody in patients with solid tumours. Br J Cancer. 126(7):1010-1017.
2. Frenay J, et al., 2022. IL-1RAP, a Key Therapeutic Target in Cancer. Int J Mol Sci. ;23(23):14918.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?