
pFUSE-Lucia-CHIg-hG4
-
Cat.code:
pfuselc-hchg4
- Documents
ABOUT
Heavy chain constant region expression plasmid - Human IgG4 - Lucia tag
pFUSE-Lucia-CHIg-hG4 is a cloning plasmid that expresses the constant region of the human IgG4 heavy chain. It contains a multiple cloning site upstream of the constant region to enable cloning of the heavy chain variable region.
This plasmid is designed for antibody generation when co-transfected into mammalian cells with the light chain variable region cloned into pFUSE-CLIg.
pFUSE-Lucia-CHIg-hG4 contain the secreted luciferase (Lucia) gene upstream of the MCS and the CH region to serve as a tag to facilitate the detection and quantification of recombinant antibodies.
All products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
Plasmid construct has been confirmed by restriction analysis and sequencing. Plasmid DNA was purified by ion exchange chromatography
CONTENTS
Contents
-
Product:pFUSE-Lucia-CHIg-hG4
-
Cat code:pfuselc-hchg4
-
Quantity:20 µg
1 ml of Zeocin™ (100 mg/ml)
Shipping & Storage
- Shipping method: Room temperature
- -20°C
Storage:
Details
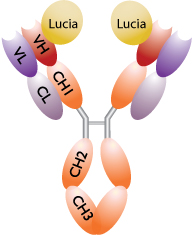
1- Schematic representation of a Lucia luciferase-tagged antibody
2- Luciferase activity of Lucia-tagged anti-hTNF-α antibodies.
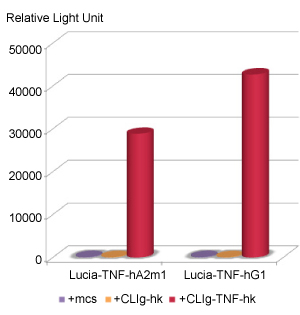
CHO cells were stably co-transfected with a heavy chain expressing plasmid, pFUSE-Lucia-TNF-CHIg-hA2m1 (TNF-CHIg-hA2m1) or pFUSE-Lucia-TNF-CHIg-hG1 (TNF-CHIg-hG1) and a light chain expressing plasmid, pFUSE2-TNF-CLIg-hk (TNF-CLIg-hk) to generate Lucia-tagged anti-hTNF-α antibodies, Lucia-anti-hTNF-α-hIgA2m1and Lucia-anti-hTNF-α-hIgG1, of human IgAm2 and human IgG1 isotypes, respectively. pFUSE2-CLIg-hk (CLIg-hk), which expresses no VL, and pSELECT-blasti-mcs (mcs) were used as negative controls. Supernatants
were collected and the luciferase levels determined using QUANTI-Luc™. Only the cells co-producing a Lucia-anti-hTNF-α heavy-chain and an anti-hTNF-α light chain displayed luciferase activity.
3- Neutralizing activity of anti-hTNF-a antibodies
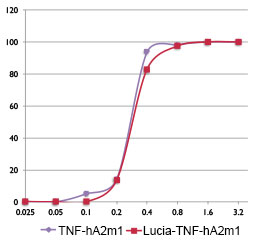
The activity of anti-hTNF-α-hIgA2m1 and Lucia-anti-hTNF-α-hIgA2m1 antibodies, purified from the supernatants of CHO transfected cells, was determined by performing an TNF-α neutralizing assay. Both antibodies display similar neutralizing activities, thus fusion of the Lucia luciferase tag at the N-terminus of the heavy chain does not alter the functionality of the antibody.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Plasmid Sequence
Certificate of analysis
Need a CoA ?
