MPLAs Vaccigrade™
-
Cat.code:
vac-mpls
- Documents
ABOUT
Synthetic lipid A
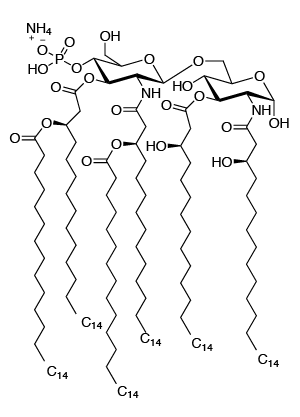
Synthetic lipid A from E. coli, serotype R515 (MPLAs) is a pure monophosphoryl lipid A compound produced by chemical synthesis. MPLAs activates TLR4 but does not activate TLR2, reflecting its high purity. MPLAs contains 6 fatty acyl groups, while MPLA purified from bacteria contains a mixture of 5, 6, and 7 acyl lipid A.
MPLA Synthetic VacciGrade™ has been tested as an adjuvant in mice and reported to induce a strong Th1 response.
MPLA Synthetic VacciGrade™ is a high-quality pre-clinical grade.
It is also available as MPLA Synthetic in a standard grade for in vitro experiments.
Background
Monophosphoryl Lipid A (MPLA) is a low-toxicity derivative of lipopolysaccharide (LPS) that retains the immunologically active lipid A portion of the parent molecule. Both LPS and MPLA are TLR4 agonists, but they signal through different adaptors, MyD88 and TRIF, respectively1, 2. The reduced toxicity of MPLA is attributed to the preferential recruitment of TRIF upon TLR4 activation, resulting in decreased induction of inflammatory cytokines. MPLA is widely used as a vaccine adjuvant due to its potent immunomodulatory properties and low inflammatory toxicity1, 2.
1. Sastry M. et al., 2017. Adjuvants and the vaccine response to the DS-Cav1stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One. 12(10):e0186854.
2. Cui W. et al., 2014. TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T Cell differentiation. J Immunol. 192(9):4221-32.
3. Steimle A. et al., 2017. Structure and function: Lipid A modifications in commensals and pathogens. Int J Med Microbiol. 306(5):290-301.
All products are for internal research use only, and not for human or veterinary use.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade, suitable for in vivo studies. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay.
SPECIFICATIONS
Specifications
TLR4
C96H184N3O22P
2- 20 μg/mouse
1 mg/ml in DMSO
0.2 µm filtration, Sterility guaranteed
Induction of TLR4 activity in cellular assays
In vivo experiments
Each lot is functionally tested and validated using cellular assays.
CONTENTS
Contents
-
Product:MPLAs Vaccigrade™
-
Cat code:vac-mpls
-
Quantity:1 mg
10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
MPLA is a low-toxicity derivative of lipopolysaccharide (LPS), that retains the immunologically active lipid A portion of the parent molecule [1]. While the toxicity associated with LPS prohibits its potential clinical use, MPLA is being developed as a vaccine adjuvant [2]. Both LPS and MPLA are TLR4 agonists, but they signal through different adaptors, MyD88 and TRIF, respectively. The reduced toxicity of MPLA is attributed to the preferential recruitment of TRIF upon TLR4 activation, resulting in decreased induction of inflammatory cytokines [3].
MPLA has been tested as an adjuvant in mice and reported to induce a strong Th1 response [4-5]. Although the mechanism of action of MPLA has not been fully eludicated, it has been suggested that MPLA improves vaccine immunogenicity by enhancing antigen presenting cell maturation [6].
1. Okemoto K. et al., 2006. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1beta or activation of caspase-1. J Immunol. 176(2):1203-8.
2. Casella CR. et al., 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 65(20):3231-40.
3. Mata-Haro V. et al., 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 316(5831):1628-32.
4. Fransen F. et al., 2007. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup. Infect Immun. 75(12) :5939-46.
5. Rhee EG. et al., 2010. TLR4 Ligands Augment Antigen-Specific CD8+ T Lymphocyte Responses Elicited by a Viral Vaccine Vector. J. Virol. 84: 10413 - 10419.
6. Didierlaurent A. et al., 2009. AS04, an aluminum salt- and TLR4 agonistbased adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 183(10): 6186-97.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?