Anti-CoV2RBD-imd-mIgG2a
-
Cat.code:
srbdc4-mab10-3
- Documents
ABOUT
Specific SARS-CoV-2 Spike-RBD recombinant human IgG1 & mouse IgG2a antibodies
Antibody description
InvivoGen provides a set of recombinant monoclonal antibodies (mAbs) featuring either a human IgG1 or a mouse IgG2a constant region, and the variable region of 'Casirivimab (REGN10933)' or 'Imdevimab (REGN10987'), two clones originally described as potent SARS-CoV-2 neutralizing mAbs [1]:
— Anti-CoV2RBD-cas-hIgG1 and Anti-CoV2RBD-cas-mIgG2a (derived from Casirivimab)
— Anti-CoV2RBD-imd-hIgG1 and Anti-CoV2RBD-imd-mIgG2a (derived from Imdevimab)
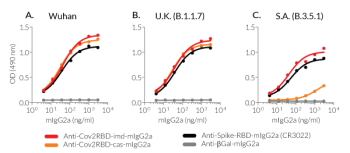
➔ These antibodies have been functionally validated by ELISA and FACS on SARS-CoV2 Spike variants.
InvivoGen's Anti-CoV2RBD-cas and Anti-CoV2RBD-imd mAbs have been generated by recombinant DNA technology, produced in CHO cells, and purified by affinity chromatography, ensuring lot-to-lot reproducibility. Furthermore, these mAbs have been functionally validated by ELISA (see data below). The absence of bacterial contamination has been confirmed by cellular assays.
Background
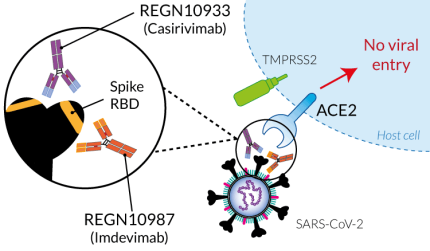
Casirivimab and Imdevimab were generated using both humanized mice and convalescent patients [1]. These mAbs specifically target two distinct and non-overlapping regions of the SARS-CoV-2 S-RBD and potently block its binding to the ACE2 receptor on target cells [1].
![]() More details on Casirivimab and Imdevimab
More details on Casirivimab and Imdevimab
Applications
- Detecting the presence of SARS-CoV-2 in culture supernatant and/or in serum (ELISA)
- Flow cytometry binding assays
- Developing neutralizing antibody cocktails against SARS-CoV-2
- Monitoring SARS-CoV-2 variant escape
Quality control
- Functionally validated by ELISA using Spike-RBD proteins derived from SARS-CoV-2 variants and either HRP or Luciferase detection
References
1. Hansen J. et al., 2020. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369:1010-1014.
All products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
ELISA, Flow cytometry, binding assays
Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
ELISA
Each lot is functionally tested by ELISA.
CONTENTS
Contents
-
Product:Anti-CoV2RBD-imd-mIgG2a
-
Cat code:srbdc4-mab10-3
-
Quantity:3 x 100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Casirivimab and Imdevimab were generated using both humanized mice and convalescent patients [1]. These mAbs specifically target two distinct and non-overlapping regions of the SARS-CoV-2 S-RBD and potently block its binding to the ACE2 receptor on target cells [1]. Treatment using a combination of these mAbs (known as REGEN-COV™ or REGN-COV2) has been authorized under emergency use for COVID-19 outpatients [2-4]. Prophylactic and therapeutic administration of this mAb cocktail almost completely blocks the establishment of virus infection in non-human primates [5] and enhances clearance of the virus in patients whose endogenous immune response has not yet been initiated [2].
Learn more about the SARS-CoV-2 Spike protein.
References
1. Hansen J. et al., 2020. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369:1010-1014.
2. Weinreich D.M. et al., 2021. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 384(3):238-251.
3. https://www.fda.gov/media/145610/download
4. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab
5. Baum A. et al., 2020. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 370:1110-1115.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?