Anti-hIL-5R-hIgG1fut
-
Cat.code:
hil5r-mab13NEW
- Documents
ABOUT
Anti-human IL-5R - Benralizumab biosimilar - CAS #1044511-01-4
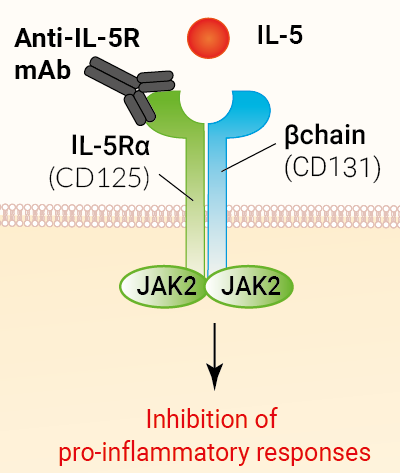
Anti-hIL-5R-hIgG1fut is a biosimilar antibody of Benralizumab, a human interleukin-5 receptor (IL-5R) antibody that blocks IL-5 signaling. This monoclonal antibody (mAb) specifically targets the alpha subunit of the IL-5R. Benralizumab, also known as MEDI-563, is FDA-approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis (EGPA).
Anti-hIL-5R-hIgG1fut comprises the variable region of Benralizumab and the afucosylated IgG1 constant region of Benralizumab for high effector functions.
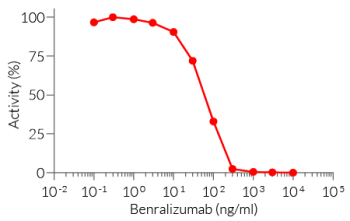
This mAb can be used together with HEK-Blue™ IL-5 cells for screening and neutralization assays to block IL-5 signaling induced by recombinant human IL-5 (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IL-5R
Human
Neutralization assay (tested)
ELISA
Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL-5R-hIgG1fut
-
Cat code:hil5r-mab13
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Benralizumab is a humanized, afucosylated IgG1 monoclonal antibody (mAb) designed to target the alpha chain of the human interleukin 5 receptor (IL-5Rα), expressed on eosinophils and basophils1. IL-5 plays a critical role in the survival, proliferation, and activation of eosinophils, which contribute to airway inflammation and exacerbations in eosinophilic asthma2. In addition to inhibiting interleukin-5 signaling, Benralizumab leads to eosinophil apoptosis through ADCC1. Benralizumab is FDA-approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis (EGPA)3.
1. Wechsler ME, et al., 2024. Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med. 390(10):911-921.
2. Kolbeck R, et al., 2010. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 125(6):1344-1353.e2.
3. Fasenra (benralizumab) US prescribing information; 2024. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761070s021lbl.pdf
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?