Anti-hIL-22-hIgG1
-
Cat.code:
hil22-mab1NEW
- Documents
ABOUT
Anti-human IL-22 - Fezakinumab biosimilar - CAS #1007106-86-6
Anti-hIL-22-hIgG1 is a biosimilar antibody of Fezakinumab, a human interleukin 22 (IL-22) antibody that blocks IL-22 signaling. This monoclonal antibody (mAb) has progressed to phase 2 clinical trials to treat patients with atopic dermatitis and rheumatoid arthritis.
Anti-hIL-22-hIgG1 comprises the variable region and the IgG1 constant region of Fezakinumab, mediating high effector functions.
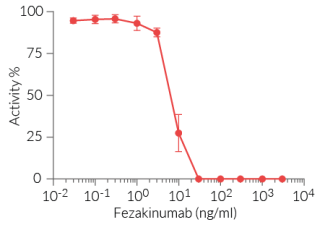
This antibody can be used together with HEK-Blue™ IL-22 cells for screening and neutralization assays to block signaling induced by recombinant human IL-22 (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IL-22
Human
Neutralization assay (tested), ELISA, ADCC
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
0.2 µm filtration
Negative (tested using EndotoxDetect™ assay)
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL-22-hIgG1
-
Cat code:hil22-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Fezakinumab background
Fezakinumab (aka ILV-094) is a therapeutic, humanized monoclonal antibody (mAb) that targets the human IL-22 (interleukin 22) [1, 2]. By binding to IL-22, Fezakinumab prevents it from interacting with its receptor on the surface of immune cells, thus inhibiting its downstream functions, notably the induction of inflammatory responses [2].
IL-22 is a member of the IL-10 cytokine superfamily. It is a key regulator of immunity and inflammation at mucosal surfaces. Its principal role is to maintain barrier integrity against pathogens [3-5]. IL-22 is implicated in several pathologies, including autoimmune diseases and cancer [5, 6].
Fezakinumab has been clinically evaluated as an immunosuppressive agent for the treatment of atopic dermatitis [2] and rheumatoid arthritis [7].
References:
1. https://drugs.ncats.io/drug/0S77U25XZ3 (accessed October 2025).
2. Guttman-Yassky, E., et al., 2018. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 78: 872.
3. Wang J. et al., 2018. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun. 24(5):297.
4. Foxall R.B., et al., 2016. Profile of interleukin-22 in gut mucosal health and disease. IJICMR. 8:1.
5. Zenewicz L.A., 2018. IL-22: There Is a Gap in Our Knowledge. Immunohorizons. 2(6):198.
6. Hernandez P., et al.,2018. A catch-22: Interleukin-22 and cancer. Eur J Immunol. 48(1):15.
7. NCT00883896. https://clinicaltrials.gov (accessed October 2025).
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?