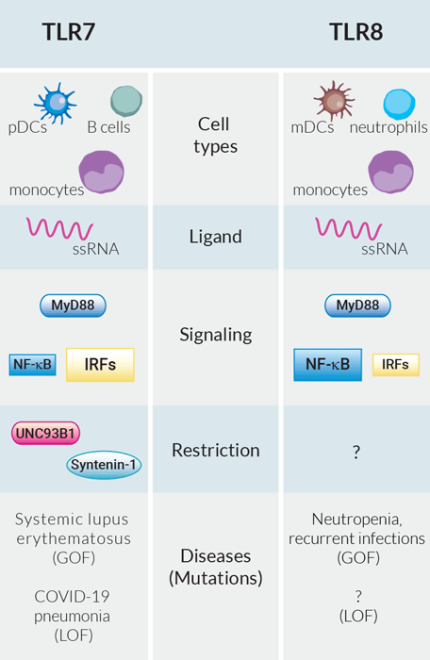
The innate immune system is armed with a limited set of germline-encoded pattern recognition receptors (PRRs) capable of sensing a tremendous variety of potential pathogens[1]. Among these PRRs, two phylogenetically and structurally highly related Toll-like receptors, TLR7 and TLR8, recognize the same ligand, single-stranded (ss) RNA[2]. Upon stimulation, they both induce the NF-κB and IRF pathways via MyD88 activating multiple immune cells (e.g. T cells), thus bridging the innate and adaptive immune response[3]. Based on these observations, it is tempting to speculate a redundant role of these two receptors, as they also share other functional homologies[1].
Despite all evidence to the contrary, TLR7 and TLR8 have evolved under strong selection, suggesting their essential and unique functions in host survival[4]. They differ in cell type-specific expression with TLR7 found in plasmacytoid dendritic cells (p)DCs and B cells, and TLR8 in myeloid (m)DCs and neutrophils. Among immune cells, only monocytes co-express both receptors[5]. While pDCs rapidly secrete large amounts of IFN-α upon IRF activation, mDCs are more involved in the NF-κB-dependent production of pro-inflammatory cytokines (e.g. IL-12, TNF-α)[5]. Moreover, both receptors contribute differently to diseases. Recently, TLR7-deficiency has been reported as a genetic mediator for severe COVID-19 pneumonia due to impaired SARS-CoV-2 detection and type I IFN production[6]. To date, no disease has yet been attributed to TLR8 loss-of-function[7]. Whether it triggers no phenotype, is lethal, or the TLR8-specific pathogen is extinct and/or yet undiagnosed remains to be elucidated. Regarding hyperactivation, improper TLR7 stimulation by self-RNA has been linked to systemic lupus erythematosus (SLE), a polygenic autoimmune disorder, characterized by the presence of self-nucleic antigens and autoantibodies[8]. Recently, the first monogenic form of SLE caused by a gain-of-function (GOF) mutation in TLR7 has been described[9]. In contrast, TLR8 GOF causes a more complex phenotype with congenital neutropenia (low level of neutrophils) and recurrent infections, but surprisingly without autoimmunity[7].
In order to distinctively dissect TLR7- and TLR8-dependent signaling, InvivoGen has developed a unique collection of HEK- and THP-1-derived cell lines, allowing the simultaneous study of the two major signaling pathways linked to TLR7/8, the NF-kB and IRF pathways. Unexpectedly, generating functional TLR7-expressing clones was extremely difficult: Whereas stable TLR7 expression was easily achieved, it was impossible to induce TLR7 activation, suggesting either missing co-factors or an underlying negative regulation of TLR7. Interestingly, this was not observed in TLR8 overexpressing cells.
A growing body of literature has highlighted the role of two key adaptor proteins UNC93B1 and syntenin-1 controlling the intracellular location, stability, and signal duration of endosomal TLRs[10, 11]. The chaperone protein UNC93B1 mediates TLR trafficking from the endoplasmic reticulum to the endosome. It specifically limits TLR7 translocation by favoring the transport of other endosomal TLRs[10]. Moreover, it remains associated with TLR7 in the endosome to stabilize it and to recruit syntenin-1. Upon TLR7 activation, syntenin-1 terminates TLR7 signaling by sorting it into intraluminal vesicles, thereby drastically reducing the half-life of functional TLR7[11]. Various mutations in UNC93B1 were described to abolish these modulatory roles and unleash TLR7[12]. Indeed, by introducing a mutant version of UNC93B1 in our TLR7-overexpressing cells, we were able to overcome the restrictive mechanism and obtain stable TLR7 signaling. The question remains why TLR7 and not TLR8 requires this strong UNC93B1-mediated regulation. Given they harbor the potential of autoreactivity to ssRNA, both their sensing certainly has to be tightly controlled. So what is restricting TLR8?
Overall, our journey of generating these cell lines perfectly illustrates the underlying complexity of TLR7/8 activity. InvivoGen's new cell lines will support scientists on their way to unravel the many mysteries surrounding TLR7 and TLR8, as well as tailor new therapeutic approaches for infectious and autoimmune diseases.
References :
Lind NA, et al., Nat Rev Immunol. 2022 Apr;22(4):224-235.
Heil F, et al., Science. 2004 Mar 5;303(5663):1526-9.
Sun H, et al., Biomark Res. 2022 Dec 7;10(1):89.
Barreiro LB, et al., PLoS Genet. 2009 Jul;5(7): e1000562.
Martínez-Espinoza I, et al., Pathogens. 2022 Jan 22;11(2):134.
Asano T, et al., Sci Immunol. 2021 Aug 19;6(62):eabl4348.
Aluri J, et al., Blood. 2021 May 6;137(18):2450-2462.
Celhar T, et al., Immunol Res. 2012 Sep;53(1-3):58-77.
Brown GJ, et al., Nature. 2022 May;605(7909):349-356.
Majer O, et al,. Nature. 2019 Nov;575(7782):366-370.
Pelka K, et al.,Immunity. 2018 May 15;48(5):911-922.e7.
Fukui R, et al., J Exp Med. 2009 Jun 8;206(6):1339-50.