Recombinant human IFN-γ
-
Human IFN-γ protein - E. coli -expressed, tag-free, with HSA
-
Cat.code:
rcyec-hifng
- Documents
ABOUT
Human IFN-γ protein - E. coli -expressed, tag-free, with HSA
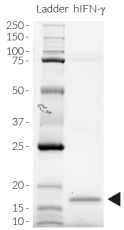
Recombinant human IFN-γ is a high-quality and biologically active cytokine, validated using proprietary IFN-γ reporter cells. This cytokine also known as type II interferon is produced in E. coli and thoroughly purified to remove endotoxins.
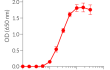
Recombinant human IFN-γ can be used together with HEK-Blue™ IFN-γ cells for the screening of inhibitory molecules, such as Fontolizumab, a monoclonal antibody that targets IFN-γ and prevents its binding to its receptor (see figures).
Key features
- Each lot is validated using HEK-Blue™ IFN-γ cells
- Endotoxin ≤ 0.1 EU/µg
- 0.2 µm sterile-filtered
Applications
- Standard for IFN-γ detection and quantification assays
- Screening and release assays for antibodies blocking IFN-γ signaling
- Screening and release assays for engineered IFN-γ
Interferon-gamma (IFN-γ) is a pro-inflammatory cytokine that plays a key role in innate and adaptive immune responses to intracellular pathogens and tumor immunosurveillance.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
P01579
100 μg/ml in water
Phosphate buffer saline (pH 7.4), 5% saccharose, 2% HSA
≤ 0.1 EU/μg (measurement by kinetic chromogenic LAL assay)
Cellular assays
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Recombinant human IFN-γ
-
Cat code:rcyec-hifng
-
Quantity:20 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
IFN-γ background
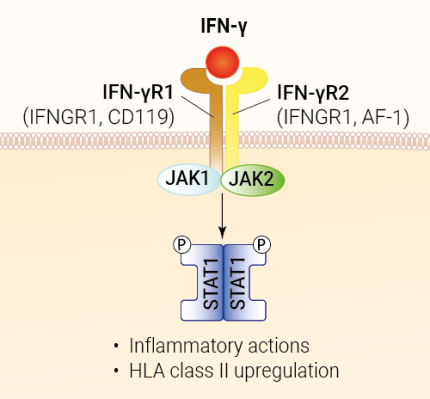
IFN-γ, also known as Type II IFN or immune interferon, is predominantly produced by innate immune cells, such as Natural Killer (NK) cells and innate lymphoid type 1 cells (ILC1), and activated adaptive immune cells, such as Th1 CD4+ T cells and cytotoxic CD8+ T cells [1]. This cytokine is produced as a secreted homodimeric molecule in response to infections and growing tumors [1, 2]. IFN-γ engages a receptor composed of two IFN-γR1 chains and two IFNγ-R2a, thus forming a hexameric complex [2]. While IFN-γR1 is constitutively expressed on all nucleated cells, the expression of IFNγ-R2 is tightly regulated. The binding of IFN-γ to its receptors triggers a JAK1/JAK2 signal transduction leading to the activation of STAT1. Activated STAT1 forms homodimers that are translocated to the nucleus where they bind interferon-gamma-activated sites (GAS) in the promoter of interferon-stimulated genes (ISGs). ISGs encode many products with direct effector or regulatory immune functions [1]. Thus IFN-γ plays a versatile role in immune responses and tissue homeostasis [1].
Relevance for therapeutics development
IFN-γ contributes to the pathogenesis of inflammatory diseases, such as Crohn's disease, psoriasis, haemophagocytic lymphohistiocytosis (HLH), and macrophage activation syndrome (MAS) [3-5].
Fontolizumab (aka HuZAF™) is a therapeutic, humanized monoclonal antibody (mAb) that targets IFN-γ. By binding to IFN-γ, Fontolizumab prevents it from interacting with its receptor (IFN-γR) on the surface of immune cells, thus inhibiting downstream inflammatory response. Fontolizumab was used as an immunosuppressive drug to treat inflammatory diseases, including Crohn's disease and psoriasis. While the mode of action was promising, clinical trials did not demonstrate sufficient clinical benefit [4, 5].
Emapalumab is a fully human monoclonal antibody (mAb) that targets both free and receptor-bound IFN-γ, preventing downstream signaling. This therapeutic antibody was FDA-approved in 2018 for treating pediatric and adult patients with HLH [3].
The administration of recombinant IFN-γ is a strategy for enhancing anti-infectious and anti-tumoral innate and adaptive immune responses.
Interferon gamma-1b (Actimmune®) is a form of recombinant human IFN-γ approved by the FDA to treat infections associated with chronic granulomatous disease and to slow the progression of severe malignant osteopetrosis [6]. IFN-γ monotherapy to treat cancer has been of limited success. This is partly explained by IFN-γ's short half-life and dual anti- and pro-tumor activities [7, 8]. Multiple clinical trials are ongoing to explore the combination of IFN-γ with other cancer therapeutics [8].
Another strategy relies on the engineering of IFN-γ partial agonists to tune IFN-γ receptor signaling output. Interestingly, such recombinant IFN-γ variants can exhibit biased gene-expression profiles, such as the retention of upregulation of class I molecules and impaired induction of inhibitory checkpoint molecules by cancer cells [2]. These results demonstrate that the two opposing functions of IFN-γ in the tumor microenvironment can be decoupled, offering a route for therapeutic applications.
References:
1. Ivashkiv L.B., 2018. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 18(9):545-558
2. Mendoza, J.L., et al., 2019. Structure of the IFNγ receptor complex guides design of biased agonists. Nature. 567(7746):56-60.
3. Vallurupalli M. & Berliner N., 2019. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 134(21):1783-1786.
4. Reinisch W et al., 2009. Fontolizumab in moderate to severe Crohn's disease: A phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Infl Bowel Dis., 16(2):233-242.
5. Harden J.L., et al., 2015. Humanized anti-IFNg (HuZAF) in the treatment of psoriasis. J. Aller Clin Immunol. 135(2):553.
6. Silva, A.C. & Lobo, J.M. Sousa., 2020. Cytokines and growth factors. Current Applications of Pharmaceutical Biotechnology. 87-113.
7. Castro, F., et al., 2018. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol. 9:847.
8. Yi, M., et al., 2024. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduction and Targeted Therapy. 9(1):176.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?