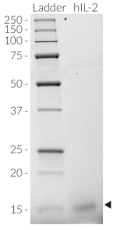
Recombinant human IL-2
-
Cat.code:
rcyc-hil2-01
- Documents
ABOUT
Human IL-2 protein - Mammalian cell-expressed, tag-free, carrier-free
Recombinant human IL-2 is a high-quality and biologically active cytokine, validated using proprietary IL-2 reporter cells. This T-cell-regulating cytokine of the γc superfamily is produced in CHO cells to ensure protein glycosylation and bona fide 3D structure.
Note: Invivogen's recombinant human IL-2 does not feature the C125S mutation of Aldesleukin (Proleukin®). This mutation, in which serine has replaced the cysteine at position 125, was introduced to stabilize the E. coli-derived cytokine [1].
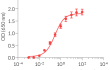
Recombinant human IL-2 can be used together with HEK-Blue™ IL-2 cells for the screening of inhibitory molecules, such as Basiliximab, a therapeutic monoclonal antibody targeting the IL-2Rα subunit of the IL-2 receptor (see figures).
Key features
- Natural wild-type human IL-2
- Each lot is validated using HEK-Blue™ IL-2 cells
- Endotoxin < 0.1 EU/µg
- 0.22 µm sterile-filtered
Applications
- T cell and NK cell activation and propagation
- IL-2 detection and quantification assays (positive control)
- Screening and release assays for antibodies blocking IL-2 signaling
- Screening and release assays for engineered IL-2 proteins
Interleukin 2 (IL-2) is a pleiotropic cytokine showing immunostimulatory or immunoinhibitory activity, depending on the target cell. In the 1990s, the recombinant IL-2 Aldesleukin became the first FDA-approved immunotherapeutic drug for treating metastatic renal cell carcinoma and metastatic melanoma.
1. Wrangle JM, et al., 2018. IL-2 and Beyond in Cancer Immunotherapy. J Interferon Cytokine Res.;38(2):45-68.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
P60568.1
>106 IU/mg*
100 μg/ml in water
Phosphate buffer saline (pH 7.4), 5% saccharose
0.22 µm filtration
The absence of bacterial contamination (e.g. lipoproteins and endotoxins) is confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Cellular assays (tested), Cell culture (T cell activation and expansion; tested), ELISA, CAR T studies
Each lot is functionally tested and validated.
*Please refer to the corresponding Certificate of Analysis (CoA) for the exact value.
CONTENTS
Contents
-
Product:Recombinant human IL-2
-
Cat code:rcyc-hil2-01
-
Quantity:10 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Interleukin 2
Interleukin 2 (IL-2) is a pleiotropic cytokine showing immunostimulatory or immunoinhibitory activity, depending on the target cell [1]. It is mainly produced by activated CD4+ T cells in response to antigen stimulation. To a lower extent, it can also be produced by CD8+ T cells and innate immune cells, such as activated dendritic cells (DCs), mast cells, monocytes, and natural killer (NK) cells [1]. IL-2 acts on lymphocytes by binding to the multimeric IL-2 receptor (IL-2R) and thereby engaging several intracellular signaling pathways that modulate lymphocyte survival, proliferation, and function [2].
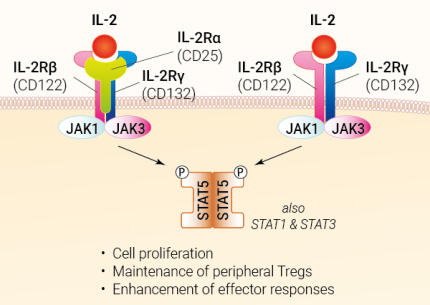
IL-2 receptor signaling
The IL2R consists of a hetero-complex of up to three subunits: α (IL-2RA), β (IL-2RB), and γ (IL-2RG), also known as CD25, CD122, and CD132, respectively. The γ subunit CD123 is also referred to as the “common” chain (labeled “γc”), as it is shared with the receptors for IL-4, IL-7, IL-9, IL-15, and IL-21 [1]. Due to its high affinity, IL-2 preferentially binds to the trimeric IL-2R, which is mainly expressed on immunosuppressive regulatory T (Treg) cells [1,3]. Interestingly, IL-2 exists in different conformations that favor either trimeric or dimeric IL-2R, resulting in the activation of different immune cells [4].
Upon receptor-ligand binding, the Janus kinase JAK3 phosphorylates JAK1, which in turn recruits SYK (spleen-associated tyrosine kinase). This leads to the phosphorylation and dimerization of the signal transducer and activator of transcription 5A/B (STAT5A/B). After its translocation to the nucleus, it regulates genes encoding immune-related factors such as IL2RA itself, Treg regulator FOXP3, or suppressor of cytokine signaling 1 or 2 (SOCS1/2). IL-2 also activates the Ras/MAPK and PI3K/Akt signaling pathways [1,5].
IL-2 therapeutic targeting
In the 1990s, recombinant IL-2 at high dosage became the first US FDA (Food and Drug Administration) approved immunotherapeutic drug for the treatment of metastatic renal cell carcinoma (RCC) and metastatic melanoma [2]. However, its adverse side effects together with its stimulatory action on immunosuppressive Tregs quickly put the brakes on the initial euphoria [3]. Combinatorial therapy of recombinant IL-2 and immune checkpoint targeting monoclonal antibodies (e.g. anti-CTLA4, PD-1) was reported to overcome these off-target effects and improve response rates [2].
Interestingly, low-dose IL-2 therapy has been shown to ameliorate autoimmune diseases and graft-versus-host disease (GvHD) [4]. Autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are caused by an imbalance between Tregs and Teffs, both of which are activated by IL-2 [6]. Selective antibodies against IL-2 can alter its conformation, thereby resulting in the selective expansion of Tregs or Teffs subsets [4,6].
The IL-2 cytokine, together with IL-7 and IL-15, is currently under investigation for application in ex vivo T cell expansion. These cytokines are used in different concentrations and combinations to improve cell viability, proliferation, and differentiation in CAR T cell culture [8].
Despite its complexity, IL-2-derived therapy remains a promising area of research, with more than 500 clinical trials listed (ClinicalTrials.gov) [7], investigating new and safer IL-2-related reagents in the fight against cancer and autoimmune diseases [2].
References:
1. Pol JG, et al., 2020. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med. 217(1):e20191247.
2. Wrangle JM, et al., 2018. IL-2 and Beyond in Cancer Immunotherapy. J Interferon Cytokine Res.;38(2):45-68.
3. Hernandez R, et al., 2022. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. (10):614-628.
4. Trotta E, et al., 2018. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med.:1005-1014.
5. Ross SH, Cantrell DA,. 2018. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu Rev Immunol.;36:411-433.
6. Yokoyama Y, et al., 2018. IL-2-Anti-IL-2 Monoclonal Antibody Immune Complexes Inhibit Collagen-Induced Arthritis by Augmenting Regulatory T Cell Functions. J Immunol. ;201(7):1899-1906.
7. Raeber et al., 2022. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. medRxiv preprint version.
8. Sudarsanam H, et al., 2022. Influence of Culture Conditions on Ex Vivo Expansion of T Lymphocytes and Their Function for Therapy: Current Insights and Open Questions. Front Bioeng Biotechnol.;10:886637
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?