Recombinant human IL-3
-
Cat.code:
rcyec-hil3
- Documents
ABOUT
Human IL-3 protein - E. coli -expressed, tag-free, carrier-free
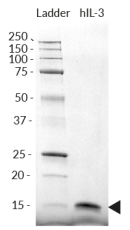
Recombinant human IL-3 is a high-quality and biologically active cytokine, validated using proprietary IL-3 reporter cells. This common β chain family member is produced in E. coli and thoroughly purified to remove endotoxins.
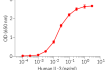
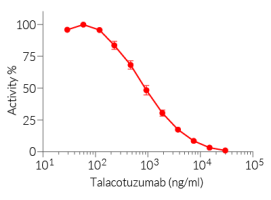
Recombinant human IL-3 can be used together with HEK-Blue™ IL-3 cells for the screening of inhibitory molecules, such as Talacotuzumab, a therapeutic monoclonal antibody targeting the IL-3Rα subunit of the IL-3 receptor (see figures).
Key features
- Each lot is validated using HEK-Blue™ IL-3 cells
- Endotoxin < 0.1 EU/µg
- 0.2 µm sterile-filtered
Applications
- Standard for IL-3 detection and quantification assays
- Screening and release assays for antibodies blocking IL-3 signaling
- Screening and release assays for engineered IL-3
Interleukin-3 (IL-3) is a cytokine that plays an important role in the recruitment, differentiation, and survival of various hematopoietic cells, especially during inflammation. It is currently regarded as a regulator of inflammation with either protective or detrimental effects in the response to infections, immune-mediated diseases, and hematologic cancers.
All products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
P08700
100 μg/ml in water
Phosphate buffer saline (pH 7.4), 8% trehalose
The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells.
Cellular assays
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Recombinant human IL-3
-
Cat code:rcyec-hil3
-
Quantity:10 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
IL-3 background
Interleukin-3 (IL-3) belongs to the common β chain (βc) cytokine family, originally identified as a multi-colony stimulation factor (CSF). It is now regarded as a regulator of inflammation [1, 2].
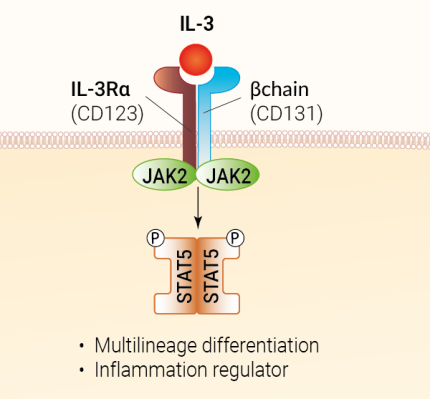
IL-3 expression is induced in response to inflammation, and is highly restricted to T cells. Under some circumstances, IL-3 may also be produced by macrophages, basophils, mast cells, NK cells and stromal cells [1, 3]. IL-3 binds a heterodimeric receptor comprising the βc (CD131) and IL-3Rα (CD123) subunits. It signals through tyrosine kinases of the Janus family (JAK2) and signal transducer and transcription activators (STATs), notably STAT5 [1, 3]. IL-3 supports the survival, proliferation, differentiation, polarization, or recruitment of immune and non-immune cells [2, 3]. Notably, as a multi-CSF, IL-3 targets a wide spectrum of hematopoietic cells, including eosinophils, basophils, plasmacytoid dendritic cells, neutrophils, and progenitor cells [3].
Relevance for therapeutics development
Depending on the clinical context, strategies have been investigated to either boost or disrupt IL-3 signaling [2, 3]. The administration of IL-3 has been evaluated as a treatment for patients with cytopenia (e.g. after chemotherapy). On the contrary, monoclonal antibodies or antibody-drug conjugates against IL-3Rα have been used in clinical trials to treat hematologic cancers. Inded, the IL-3Rα subunit of the IL-3 receptor is overexpressed in acute myeloid lymphoma (AML), blastic plasmacytoid dendritic cell neoplasm (BPDCN), B-cell acute lymphoblastic leukemia, or Hodgkin lymphoma [2, 3].
Talacotuzumab (CSL362) is a monoclonal antibody targeting the IL-3Rα subunit, and exhibiting a modified Fc region for enhanced ADCC functions. Thus, it disrupts the IL-3 signaling and kills the malignant cells that overexpress IL-3Rα [4]. Although Talacotuzumab demonstrated promising activity in AML patients [5], later phase 2/3 clinical studies pointed to considerable toxicity [6].
References:
1. Dougan, M. et al., 2019. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 50(4):796-811.
2. Podolska, M.J. et al., 2024. IL-3: key orchestrator of inflammation. Front Immunol. 15:1411047.
3. Pant, H. et al., 2023. Translating the biology of β common receptor-engaging cytokines into clinical medicine. J. Allergy & Clin Immunol. 151(2):324-344.
4. Busfield, S.J. et al., 2014. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia. 28(11):2213-2221.
5. Xie, L.H. et al., 2017. CD123 target validation and preclinical evaluation of ADCC activity of anti-CD123 antibody CSL362 in combination with NKs from AML patients in remission. Blood Cancer J 7(6):e567.
6. Montesinos P., et al., 2021. Safety and efficacy of talacotuzumab plus decitabine or decitabine alone in patients with acute myeloid leukemia not eligible for chemotherapy: results from a multicenter, randomized, phase 2/3 study. Leukemia 35, 62–74.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?