THP1-KO-NLRC4 Cells
-
Cat.code:
thp-konlrc4z
- Documents
ABOUT
NLRC4 knockout in THP-1 cells
NLRC4 (Nucleotide-binding domain (NBD) and leucin-rich repeat (LRR) receptor, CARD domain-containing protein 4, or IPAF) is a cytoplasmic protein which upon activation, triggers the assembly of an NLRC4 inflammasome [1-3].
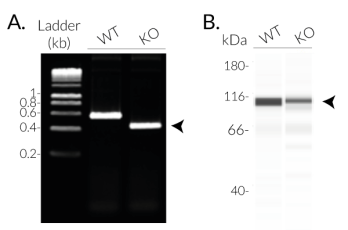
InvivoGen has developed THP1-KO-NLRC4 cells, a human monocytic cell line derived from THP1-Null2 cells through the deletion of the critical NBD region in the NLRC4 gene. NBD is essential for the NLRC4 polymerization and inflammasome assembly [1]. Thus THP1-KO-NLRC4 cells feature an inactive NLRC4 protein, unable to form a functional inflammasome.
• THP1-KO-NLRC4 cells – Knockout (KO) of the Nucleotide-binding domain (NBD) region of the NLRC4 gene
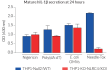
These cells exhibit impaired IL-1β secretion and pyroptosis upon incubation with known inducers of the NLRC4 inflammasome. Importantly, IL-1β secretion and pyroptosis remain unaffected upon incubation with other inflammasome inducers such as Nigericin (NLRP3 inducer).
Features of THP1-KO-NLRC4 cells:
- Absence of functional NLRC4 protein expression
- Verified biallelic knockout of the NOD region in the NLRC4 gene (DNA sequencing, PCR, and Western blot)
- Altered IL-1β secretion and pyroptosis early upon NLRC4 inflammasome activation
For the detection and quantification of mature human (h)IL-1β release, InvivoGen provides HEK-Blue™ IL-1β sensor cells, which express an NF-κB-inducible SEAP reporter gene. QUANTI-Blue™ Solution allows rapid colorimetric detection and measure of SEAP activity by reading the optical density at 630-650 nm.
![]() Download our Practical guide on Inflammasomes
Download our Practical guide on Inflammasomes
References:
1. Zhang L. et al., 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 350:404-409.
2. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477: 596-600.
3. Bauer R. & Rauch I., 2011. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020 Jun 1:100863.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
Complete RPMI 1640 (see TDS)
Validated using PlasmotestTM.
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:THP1-KO-NLRC4 Cells
-
Cat code:thp-konlrc4z
-
Quantity:3-7 x 10^6 cells
- 1 ml of Zeocin® (100 mg/ml).
- 1 ml of Normocin™ (50 mg/ml).
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
Storage:
Details
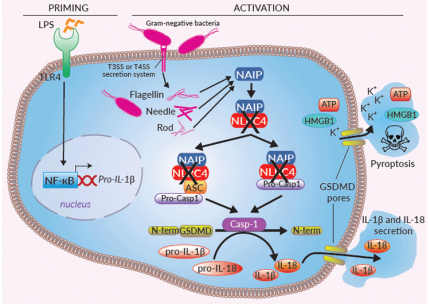
Inflammasomes are cytoplasmic multi-protein complexes, characterized by a primary sensor, that assemble in response to infections and cellular damage. NLRC4 is an indirect sensor that must associate with NAIP (NLR family apoptosis inhibitory protein) to induce the assembly of an NLRC4 inflammasome. A single NAIP operates upstream of NLRC4 in humans and recognizes intracellular Flagellin, Needle, or Rod from several Gram-negative bacterial strains [1]. These ligands are sensed by NAIP upon bacterial invasion, or cytosolic translocation through the bacterial type III or IV secretion systems (T3SS or T4SS).
Once recruited by NAIP, NLRC4 triggers a homo-polymerization through NBD-NBD interactions, allowing a CARD clustering [1]. The NLRC4 polymer further associates to pro-caspase-1, either through direct CARD-CARD interaction or through the binding of the ASC (apoptosis-associated speck-like protein) adaptor [1]. Activation of caspase-1 induces the maturation of pro-IL-1β/pro-IL-18, cleavage of the pore-forming gasdermin D (GSDMD), secretion of IL-1β/-18, and pyroptosis [1-3]. The NLRC4 inflammasome appears to protect mucosal barriers such as the lung, stomach, and intestine from invading bacteria [3]. Gain-of-function mutations have been described in human NLRC4 and are associated with auto-inflammatory conditions [3].
1. Zhang L. et al., 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 350:404-409.
2. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477: 596-600.
3. Bauer R. & Rauch I., 2011. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020 Jun 1:100863.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?