THP1-KO-NLRP3 Cells
-
Cat.code:
thp-konlrp3z
- Documents
ABOUT
NLRP3 knockout in human monocytes for inflammasome studies
THP1-KO-NLRP3 cells are designed to foster research on the NLRP3 (NOD-like receptor pyrin domain-containing protein 3, cryopyrin or NALP3) inflammasome sensor. These cells feature a knockout (KO) of the human NLRP3 gene; thus, they have no functional NLRP3 protein expression. THP-1 monocytes are widely used for inflammasome studies due to their high expression levels of NLRP3, ASC, and pro-caspase 1.
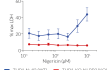
In THP1-KO-NLRP3 cells, mature IL-1β secretion is abolished following stimulation with NLRP3 inflammasome inducers (e.g., Nigericin, MSU crystals, Alum), compared to their parental cell line THP1-Null2. Furthermore, NLRP3-driven pyroptosis is absent in NLRP3-KO cells, as confirmed by the LDH-Blue™ cytotoxicity assay (see figures).
THP1-KO-NLRP3 cells are a valuable tool for investigating the role of the NLRP3 sensor in inflammasome responses. They can also function as a control cell line for the screening of novel therapeutics that target NLRP3 signaling pathways.
Key features
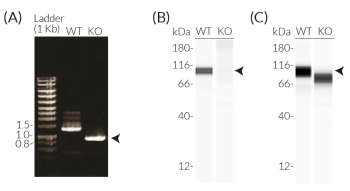
- Verified biallelic KO of the N-terminal region of the NLRP3 gene
- Complete abrogation of mature IL-1β secretion or pyroptosis
- Convenient readout using IL-1β HEK 293 reporter cells
- Stability guaranteed for 20 passages
NLRP3 (NOD-like receptor pyrin domain-containing protein 3, cryopyrin or NALP3) is a cytoplasmic protein and the best-described inflammasome sensor [1, 2]. The inflammasome response requires two signals: priming (recognition of PAMPs or DAMPs by pattern recognition receptors such as TLRs) and activation. Activation of NLRP3 can be induced by a wide range of stimuli (e.g. Nigericin, ATP, crystals), and instead of directly binding to them, NLRP3 senses downstream cytosolic stress signals such as ion imbalances (i.e., K+ efflux).
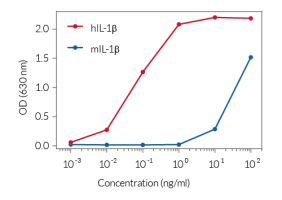
To detect and quantify mature human IL-1β release, InvivoGen provides HEK-Blue™ IL-1β sensor cells, engineered to express an NF-κB-inducible SEAP reporter gene. SEAP activity can be rapidly measured using QUANTI-Blue™ Solution through the colorimetric readout at 650 nm.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
NLRP3
Human
Cellular assays using NLRP3 agonists
Complete RPMI 1640 (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:THP1-KO-NLRP3 Cells
-
Cat code:thp-konlrp3z
-
Quantity:3-7 x 10^6 cells
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
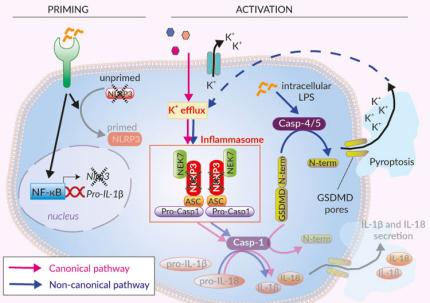
Inflammasomes are cytoplasmic multi-protein complexes that assemble in response to infections and cellular damage. Canonical and non-canonical inflammasomes have been identified. Canonical inflammasomes are characterized by a primary sensor, such as NLRP3, that recruits the ASC adaptor leading to caspase-1 (CASP1) activation.
The canonical inflammasome response requires two signals, priming (recognition of PAMPs or DAMPs by pattern recognition receptors such as TLRs) and activation [1,2]. Activation of NLRP3 can be triggered by a wide range of structurally and chemically unrelated stimuli (e.g. pore-forming toxins, activators of ion channels, MSU crystals, β-amyloid proteins). Therefore, instead of directly binding to these stimuli, NLRP3 senses downstream cytosolic stress signals such as ion imbalances (e.g. K+ efflux) [2]. This leads to the aggregation of NLRP3 and the ASC adaptor and the cleavage and activation of CASP1. This induces the maturation of pro-IL-1β/pro-IL-18, and cleavage of the pore-forming protein gasdermin D (GSDMD), leading to the secretion of IL-1β and IL-18 as well as pyroptosis [1,2].
Additionally, NLRP3 is activated indirectly by the induction of the non-canonical inflammasome (CASP4/5 in humans and CASP11 in mice) upon the sensing of cytosolic LPS. These caspases trigger GSDMD‑driven release of alarmins and K+ efflux, which ultimately induces the activation of NLRP3 and CASP1-mediated IL-1β/-18 maturation and secretion [1,2].
1. Swanson K.V. et al., 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19:477.
2. Groslambert M. & Py B. 2018. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 11:359.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?