HEK-Dual™ hTLR8 Cells
-
Cat.code:
hkd-htlr8
- Documents
ABOUT
NF-κB–SEAP and IRF–Lucia reporter HEK293 cells expressing human TLR8
HEK-Dual™ hTLR8 cells were engineered from HEK-Dual™ cells, a human embryonic kidney HEK293-derived cell line, to study the human Toll-like receptor 8 (hTLR8). This important pattern recognition receptor (PRR) recognizes - together with TLR7 - single-stranded (ss)RNA structures, a hallmark of viral replication, and triggers antiviral immune responses [1].
Description
HEK-Dual™ hTLR8 cells feature the stable expression of the TLR8 gene as well as two inducible reporter genes for SEAP (secreted embryonic alkaline phosphatase) and Lucia luciferase. As a result, these cells allow the simultaneous study of the NF-κB pathway, by monitoring the activity of SEAP, and the IRF pathway, by assessing the activity of the secreted Lucia luciferase. Upon TLR8 activation, both reporter proteins are readily measurable in the cell culture supernatant when using QUANTI-Blue™ Solution, a SEAP detection reagent, and QUANTI-Luc™ 4 Lucia/Gaussia, a Lucia luciferase detection reagent.
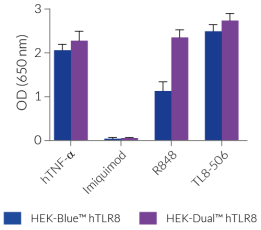
HEK-Dual™ hTLR8 cells show potent NF-κB and IRF responses upon incubation with TLR8-specific ligands, such as TL8-506 when compared to HEK-Blue™ hTLR8 cells and their parental cell line HEK-Dual™ (see figures). They do not respond to TLR7-specific ligands, since HEK293 cells do not express endogenous TLR7. However, as they express endogenous levels of various PRRs, such as TLR3 and TLR5 [in-house data], HEK-Blue™ hTLR8 cells may respond to their cognate ligands Poly(I:C) and flagellin (see figures).
Key features:
- Stable expression of human TLR8
- Strong response to TLR7/8- and TLR8-specific ligands
- Distinct monitoring of TLR8-dependent NF-κB or IRF activation by assessing the SEAP and Lucia luciferase activities
Applications:
- Defining the role of TLR8-dependent IRF and NF-κB signaling pathways
- Screening for novel TLR8 agonists and inhibitors
References
1. Heil F. et al., 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 303:1526.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR8
Human
Dual reporter assays for TLR8 pathway, NF-κB and IRF signaling pathways
Complete DMEM (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Dual™ hTLR8 Cells
-
Cat code:hkd-htlr8
-
Quantity:3-7 x 10^6 cells
- 1 ml Blasticidin (10 mg/ml)
- 1 ml Puromycin (10 mg/ml)
- 1 ml Zeocin® (100 mg/ml)
- 1 ml Normocin™ (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution)
- 1 tube of QUANTI-Luc™ 4 Reagent (sufficient to prepare 25 ml)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Toll-Like Receptor 8
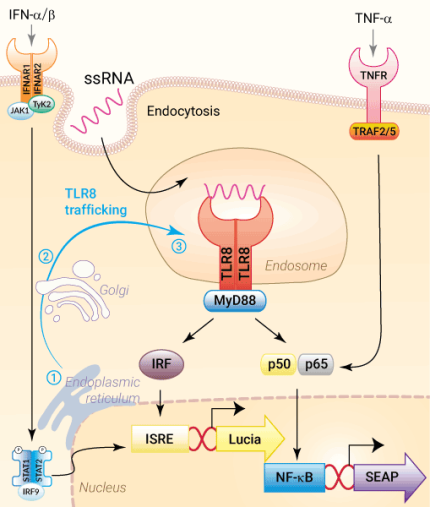
In humans, four Toll-Like Receptor (TLR) family members TLR3, TLR7, TLR8, and TLR9, mainly found in the endosome, are specialized in sensing viral-derived components. TLR7 and TLR8 recognize single-stranded (ss)RNA structures, such as viral ssRNA, miRNA, and various synthetic agonists [1]. Despite their similarities in PAMP (pathogen-associated molecular pattern) recognition, structure, and signaling partners, they highly differ in expression profiles and signaling responses, with TLR7 being more involved in the antiviral immune response and TLR8 mastering the production of proinflammatory cytokines [2]. TLR7 is mainly found in plasmacytoid dendritic cells (pDCs) and B cells, whereas TLR8 is highly expressed in monocytes, monocyte-derived DCs (mDCs), and macrophages [3].
TLR8 signaling
Upon viral infection, TLR8 translocates from the endoplasmic reticulum via the Golgi into the endosomes. Subsequently, it undergoes proteolytic cleavage in order to facilitate dimer rearrangement [1,3]. While TLR7 dimerizes upon ligand binding, TLR8, which exists as an unliganded inactive dimer, performs structural reorganization after ligand recognition [4]. Once activated, TLR8 recruits the adaptor protein MyD88 to trigger IRF, AP-1, and NF-kB responses via TRAF6 (TNF receptor-associated factor 6) [1,3]. Depending on the stimulus and cell type, TLR8-mediated signaling induces Th1-type cytokine (IL-12) production [5].
TLR8 therapeutic targeting
The involvement of nucleic acid-sensing mechanisms in the immune response against infections and other diseases makes them interesting targets for drug design [5]. TLR7/8 agonists are currently been tested as vaccine adjuvants and immunomodulatory therapeutics. They are extensively studied in the context of viral infection (e.g. SARS-CoV-2, Influenza, HIV), autoimmune (e.g. asthma, Lupus), and autoinflammatory diseases (e.g. cancer) [1-5]. Understanding the fundamental differences between these two related receptors could potentially be harnessed to discover novel drugs and improve vaccine efficacy/safety [5].
References:
1. Martínez-Espinoza I & Guerrero-Plata A. 2022. The Relevance of TLR8 in Viral Infections. Pathogens. 11(2):134.
2. Salvi V, et al., 2021. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight.;6(18):e150542.
3. Georg P. & Sander L.E., 2019. Innate sensors that regulate vaccine responses. Curr. Op. Immunol. 59:31.
4. Asami J, Shimizu T. 2021. Structural and functional understanding of the toll-like receptors. Protein Sci. (4):761-772.
5. de Marcken M, et al., 2019. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal.;12(605):eaaw1347.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?