HEK-Blue-Lucia™ hTLR9 Cells
-
Cat.code:
hkd-htlr9ni
- Documents
ABOUT
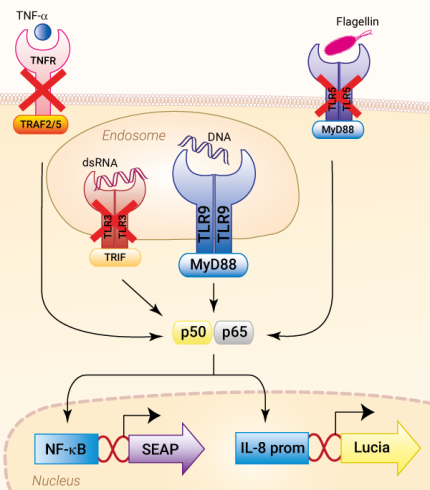
NF-κB–SEAP and IL-8–Lucia reporter HEK293 cells expressing human TLR9
HEK-Blue-Lucia™ hTLR9 cells* were engineered from the human embryonic kidney 293 (HEK293)-derived cell line, specifically designed to assess the distinct role of the human Toll-like receptor 9 (hTLR9). TLR9 is an important pattern recognition receptor (PRR) that recognizes unmethylated CpG dinucleotides, a hallmark of microbial (bacterial, viral, fungal, and parasite) as well as mitochondrial self-DNA.
Description
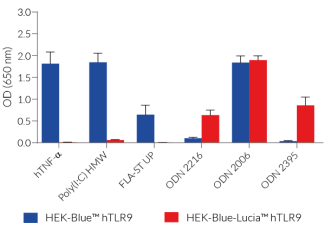
HEK-Blue-Lucia™ hTLR9 cells were generated from the HEK-Blue-Lucia™ Null cell line to stably express the human TLR9 gene, as well as NF-κB/AP-1-inducible SEAP and IL-8-inducible Lucia® reporter genes. IL-8 (interleukin 8) is a chemokine produced in response to TLR agonists in an NF-κB/AP-1-dependent manner [1, 2]. This feature enables a double readout of the NF-κB/AP-1 activation, upon incubation with TLR9 agonists, including CpG ODNs, by monitoring the production of SEAP and Lucia® luciferase, using QUANTI-Blue™ Solution and QUANTI-Luc™ 4 Lucia/Gaussia, respectively (see figures).
Of note, the parental cell line HEK-Blue-Lucia™ Null may respond slightly to TLR9-specific agonists due to the weak endogenous expression of TLR9 in HEK293 cells (data not shown).
Key features
- Stable overexpression of human TLR9
- Strong response to human-preferred CpG ODN
- Unresponsive to TLR3, TLR5, and TNFR stimulation
- Readily assessable NF-κB activation by assessing the SEAP and/or Lucia® luciferase activities
- Provided with Normocin®, an antibiotic formulation active against mycoplasmas, bacteria, and fungi
Applications
- Defining the role of the TLR9-dependent NF-κB signaling pathway
- Screening for novel TLR9 agonists and inhibitors
*Note: This cell line has been renamed. It was formerly known as "HEK-Dual™ hTLR9 (NF/IL8)". The cat. code (hkd-htlr9ni) remains unchanged.
1. Roebuck KA. 1999. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res:429-38.
2. Ohta K, et al. 2014. Toll-like receptor (TLR) expression and TLR‑mediated interleukin-8 production by human submandibular gland epithelial cells. Mol Med Rep. (5):2377-82.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR9
Human
Detection and quantification of human TLR9 activity
Complete DMEM (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Blue-Lucia™ hTLR9 Cells
-
Cat code:hkd-htlr9ni
-
Quantity:3-7 x 10^6 cells
- 1 ml of Hygromycin B Gold (100 mg/ml)
- 1 ml of Zeocin® (100mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Toll-Like Receptor 9 (TLR9) is an endosomal receptor that triggers NF-κB- and IRF-mediated pro-inflammatory responses upon the recognition of unmethylated cytosine-phosphorothioate-guanosine (CpG) forms of DNA [1-3]. Unmethylated CpG dinucleotides are a hallmark of microbial (bacterial, viral, fungal, and parasite) DNA, as well as mitochondrial self-DNA [3,4]. These TLR9 agonists can be mimicked by synthetic oligonucleotides containing CpG motifs (CpG ODNs), which have been extensively studied to improve adaptive immune responses in the context of vaccination [1,3].
TLR9 is mainly expressed in subsets of Dendritic Cells and in B cells of all mammals. In rodents, but not in humans, TLR9 is also expressed in monocytes and macrophages [3]. The structure of the receptor varies by 24% between human TLR9 (hTLR9) and mouse TLR9 (mTLR9) [3]. They recognize different CpG motifs, the optimal sequences being GTCGTT and GACGTT for hTLR9 and mTLR9, respectively [5].
![]() Get more information about CpG-ODNs Classes.
Get more information about CpG-ODNs Classes.
References
1. Kumagai Y. et al., 2008. TLR9 as a key receptor of the recognition of DNA. Adv. Drug. Deliv. Rev. 60(7):795-804.
2. Heinz L.X. et al., 2021. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature. 581(7808):316-322.
3. Kayraklioglu N. et al., 2021. CpG oligonucleotides as vaccine adjuvants. DNA Vaccines: Methods and Protocols. Methods in Molecular Biology. Vol. 2197. p51-77.
4. Kumar V., 2021. The trinity of cGAS, TLR9, and ALRs: guardians of the cellular galaxy against host-derived self-DNA. Front. Immunol. 11:624597.
5. Bauer S. et al., 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA, 98(16):9237-42.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?