HEK-Blue-Lucia™ hTLR3 Cells
-
Cat.code:
hkd-htlr3ni
- Documents
ABOUT
NF-κB–SEAP and IL-8–Lucia reporter HEK293 cells expressing human TLR3
HEK-Blue-Lucia™ hTLR3 cells* were engineered from the human embryonic kidney 293 (HEK293)-derived cell line, specifically designed to assess the distinct role of the human Toll-like receptor 3 (hTLR3). Upon recognition of viral or synthetic dsRNA, TLR3 mediates the transcriptional induction of type I interferons (IFNs), proinflammatory cytokines, and chemokines, thereby collectively establishing an antiviral host response [1].
Description
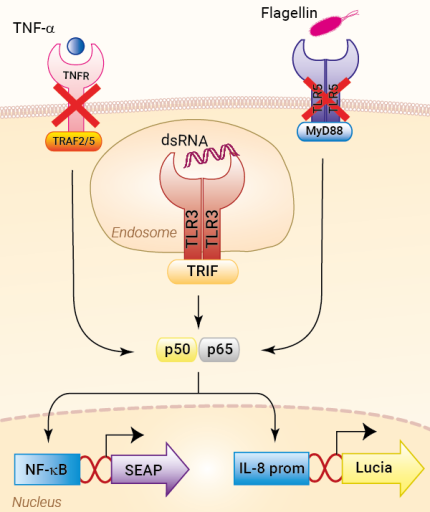
HEK-Blue-Lucia™ hTLR3 cells were generated from the HEK-Blue-Lucia™ Null cell line harboring two inducible reporter genes. This feature allows the double readout of the NF-κB/AP-1 pathway, by monitoring the SEAP (secreted embryonic alkaline phosphatase) or Lucia luciferase activities. These cells stably express the human TLR3 gene. Moreover, they are unresponsive to TLR5 and TNFR stimulation.
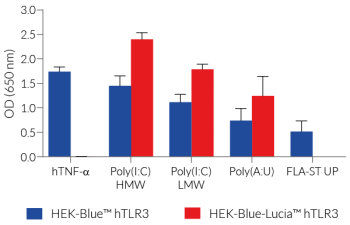
Stimulation of HEK-Blue-Lucia™ hTLR3 cells with TLR3 agonists (e.g. Poly(I:C)) triggers the activation of the artificial NF-κB-inducible promoter and the subsequent production of SEAP. It also promotes the expression of Lucia luciferase, which is knocked in (KI) downstream of the endogenous (more physiological) IL-8 promoter (see figures).
IL-8 (interleukin 8) is a chemokine produced in response to TLR agonists in an NF-κB/AP-1-dependent manner [2-3]. This feature enables the double readout study of the NF-κB/AP-1 pathway, by monitoring the activity of SEAP and Lucia luciferase using QUANTI-Blue™ Solution (SEAP detection reagent) or QUANTI-Luc™ 4 Lucia/Gaussia (luciferase detection reagent), respectively. Thus, you may choose the readout depending on your laboratory equipment utilizing a spectrophotometer for SEAP or a luminometer for Lucia luciferase detection.
Key features
- Stable expression of human TLR3
- Strong response to dsRNA and analogs
- Unresponsive to TLR5 and TNFR stimulation
- Readily assessable NF-κB activation by assessing the SEAP and/or Lucia luciferase activities
Applications
- Defining the role of TLR3-dependent NF-κB signaling pathway
- Screening for novel TLR3agonists and inhibitors
- Choice of readout depending on the laboratory equipment (spectrophotometer for SEAP or luminometer for Lucia luciferase detection).
* Note: This cell line has been renamed. It was formerly known as "HEK-Dual™ hTLR3 (NF/IL8)". The cat. code (hkd-htlr3ni) remains unchanged.
References:
1. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
2. Roebuck KA. 1999. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res:429-38.
3. Ohta K, et al. 2014. Toll-like receptor (TLR) expression and TLR‑mediated interleukin-8 production by human submandibular gland epithelial cells. Mol Med Rep. (5):2377-82.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR3
Human
TLR3 activation cellular assays
Complete DMEM (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Blue-Lucia™ hTLR3 Cells
-
Cat code:hkd-htlr3ni
-
Quantity:3-7 x 10^6 cells
- 1 ml of Hygromycin B Gold (100 mg/ml)
- 1 ml of Zeocin® (100mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
Storage:
Details
In humans, four Toll-Like Receptor (TLR) family members TLR3, TLR7, TLR8, and TLR9 are specialized in sensing viral-derived components and are mainly found in the endosome. Among these, TLR3 recognizes double-stranded (ds)RNA, a hallmark of viral replication, and triggers antiviral immune responses [1]. TLR3 is expressed in myeloid dendritic cells, macrophages, as well as non-immune cells [2].
TLR3 activation upon viral infection involves several steps, including translocation of TLR3 from the ER (endoplasmic reticulum) via the Golgi to the endosome, proteolytic cleavage and dimerization of TLR3, and finally receptor-ligand binding [3]. In order to start the signaling cascade, activated TLR3 recruits the adaptor protein TRIF (TIR domain-containing adapter-inducing interferon-β). TRIF binds to TRAF3 (TNF receptor-associated factor 3), which then recruits TBK1 (TANK-binding kinase 1) and IKKε (IκB kinase ε), thus activating the transcription factor IRF3 (interferon regulatory factor 3) and stimulating the production of type I IFNs (interferons). Additionally, TRIF interacts with TRAF6 and RIP1 (kinase receptor-interacting protein 1). RIP1 in turn binds to TAK1 (transforming growth factor β-activated kinase 1) and IKK. TAK1 phosphorylates IKKα and IKKβ, leading to the phosphorylation of IκB, the NF-κB inhibitor. Ultimately, this leads to the release and translocation of NF-κB into the nucleus and the induction of pro-inflammatory cytokines [2,4].
Given its important role in dsRNA recognition, TLR3 signaling has been intensively studied. Various TLR3-agonists, such as the synthetic dsRNA analog Poly(I:C) are being used in vaccine development and cancer therapy [4]. Yet, recent studies have indicated that TLR3 may act as a double-edged sword by showing both protective and damaging functions in the context of some human viral infections [3,5]. Moreover, rare mutations in TLR3 have been associated with viral susceptibility; specifically, infections with HSV-1 (herpes simplex virus 1), influenza, and SARS-Co-V2 have been linked to pathogenic germline variants in TLR3 pathway genes [2]. Understanding the TRIF-dependent TLR3 pathway may be essential for the establishment of specific therapeutic approaches to diminish TLR3-driven disease and exploit its protective functions [3].
References
1. Manuela Sironi, et al., 2012. A Common Polymorphism in TLR3 Confers Natural Resistance to HIV-1 Infection. J Immunol 15; 188 (2): 818–823.
2. Aluri, J, et al., 2021. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells, 10, 1374.
3. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
4. Komal A, et al., 2021. TLR3 agonists: RGC100, ARNAX, and poly-IC: a comparative review. Immunol Res. 69(4):312-322.
5. Perales-Linares R, Navas-Martin S. 2013. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology.;140(2):153-67.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?