HEK-Blue-Lucia™ hTLR5 Cells
-
Cat.code:
hkd-htlr5ni
- Documents
This cell line has been renamed. It was formerly known as "HEK-Dual™ hTLR5 (NF/IL8)". The cat. code (hkd-htlr5ni) remains unchanged.
ABOUT
NF-κB–SEAP and IL-8–Lucia reporter HEK293 cells expressing human TLR5
InvivoGen offers a human embryonic kidney 293 (HEK293)-derived cell line, specifically designed to assess the distinct role of the human Toll-like receptor 5 (hTLR5):
— HEK-Blue-Lucia™ hTLR5 cells*
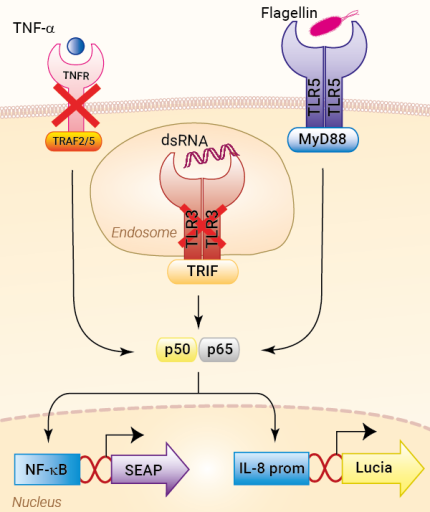
These cells were generated from the HEK-Blue-Lucia™ Null cell line harboring two inducible reporter genes. This feature allows the double readout of the NF-κB/AP-1 pathway, by monitoring the SEAP (secreted embryonic alkaline phosphatase) or Lucia luciferase activities. HEK-Blue-Lucia™ hTLR5 cells also stably express the hTLR5 gene. Due to a double knockout (KO) of TLR3 and TNFR, this cell line allows for the independent study of TLR5.
Stimulation of HEK-Blue-Lucia™ hTLR5 cells with TLR5 agonists (e.g. flagellin) triggers the activation of the artificial NF-κB-inducible promoter and the subsequent production of SEAP. It also promotes the expression of Lucia luciferase, which is knocked in (KI) downstream of the endogenous (more physiological) IL-8 promoter (see figures).
IL-8 (interleukin 8) is a chemokine produced in response to TLR agonists in an NF-κB/AP-1-dependent manner [1-2]. This feature enables the double readout study of the NF-κB/AP-1 pathway, by monitoring the activity of SEAP and Lucia luciferase using QUANTI-Blue™ Solution (SEAP detection reagent) or QUANTI-Luc™ 4 Lucia/Gaussia (luciferase detection reagent), respectively. Thus, you may choose the readout depending on your laboratory equipment utilizing a spectrophotometer for SEAP or a luminometer for Lucia luciferase detection.
Upon recognition of the flagellin of Gram-negative or -positive bacteria, TLR5 mediates the transcriptional induction of proinflammatory cytokines, such as TNF-α [3].
Key features:
- Stable overexpression of hTLR5
- Verified KO for the TLR3 and TNFR genes
- Functionally validated using a selection of PRR ligands and cytokines
- Readily assessable NF-κB activation by assessing the SEAP and/or& Lucia luciferase activities
Applications:
- Defining the role of TLR5-dependent NF-κB signaling pathway
- Screening for novel TLR5 agonists and inhibitors
- Choice of readout depending on the laboratory equipment (spectrophotometer for SEAP or luminometer for Lucia luciferase detection).
* Note: This cell line has been renamed. It was formerly known as "HEK-Dual™ hTLR5 (NF/IL8)". The cat. code (hkd-htlr5ni) remains unchanged.
References:
1. Roebuck KA. 1999. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res:429-38.
2. Ohta K, et al. 2014. Toll-like receptor (TLR) expression and TLR‑mediated interleukin-8 production by human submandibular gland epithelial cells. Mol Med Rep. (5):2377-82.
3. Yoon SI et al. 2012. Structural basis of TLR5-flagellin recognition and signaling. Science. ;335(6070):859-64.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR5 activation cellular assays
Complete DMEM (see TDS)
Verified using PlasmotestTM.
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Blue-Lucia™ hTLR5 Cells
-
Cat code:hkd-htlr5ni
-
Quantity:3-7 x 10^6 cells
- 1 ml of Hygromycin B Gold (100 mg/ml)
- 1 ml of Zeocin® (100mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer
- 1 tube of QUANTI-Luc™ 4 Reagent
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
Storage:
Details
The Toll-like receptor 5 (TLR5) is an important pattern recognition receptor (PRR) of the innate immune system playing an essential role in the respiratory tract, gastrointestinal tract, and liver. It recognizes a wide variety of pathogen-associated molecular patterns (PAMPs), specifically flagellin, the major structural protein of Gram-positive and Gram-negative bacterial flagella [1]. TLR5 is expressed constitutively in epithelial cells and immune cells, such as monocytes and immature dendritic cells (DCs). It is preferentially found on the apical side of respiratory epithelia in both mice and humans [1]. Activation of the receptor stimulates the production of proinflammatory cytokines, such as TNF-α, through signaling via the adaptor protein MyD88 [1-3]. TLR5 can generate a pro-inflammatory signal as a homodimer suggesting that it might be the only TLR participating in flagellin recognition [3]. However, TLR5 may require the presence of a co-receptor or adaptor molecule for efficient ligand recognition and/or signaling [4].
References
1. Yang J. & Yan H. 2017. TLR5: beyond the recognition of flagellin.Cell Mol Immunol. 14(12):1017-1019.
2. Gewirtz AT. et al., 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol, 167(4):1882-5.
3. Hayashi F. et al., 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature, 410(6832):1099-103.
4. Tallant T. et al., 2004. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 4(1):33.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?