QS-21 VacciGrade™
-
Cat.code:
vac-qs21-1
- Documents
ABOUT
Saponin vaccine adjuvant formulation of STIMULON® QS-21 from cultured plant cells – Sterile
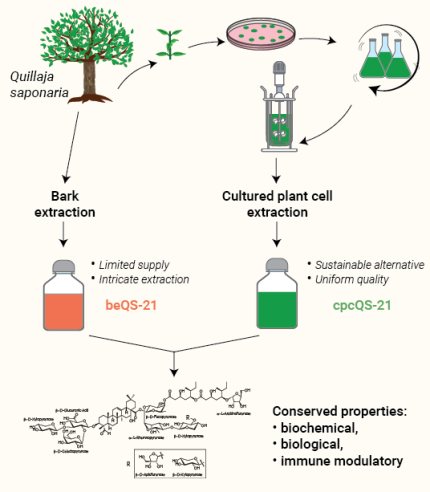
InvivoGen provides QS-21 VacciGrade™, a sterile and potent vaccine adjuvant obtained from plant cell culture of authenticated native Quillaja saponaria trees [1]. This innovative cultured plant cell production of QS-21 (cpcQS-21) offers a uniform quality and a sustainable alternative to conventional QS-21 extracted from the tree bark (beQS-21). Indeed, the limited cultivation of Chilean soapbark trees Quillaja saponaria, coupled to the intricate and costly bark extraction, makes conventional QS-21 (STIMULON® beQS-21) a scarce and expensive adjuvant.
Importantly, STIMULON® cultured plant cell QS-21 and STIMULON® bark extract QS-21 feature conserved chemical, biological, and immune modulatory properties [1].
QS-21 is a potent vaccine adjuvant and a key component of several licensed human vaccines. Notably, STIMULON® beQS-21 is used in adjuvant systems such as AS01®, a liposomal mixture of QS-21 and a TLR4 agonist, 3-O-desacyl-4'-monophosphoryl lipid A (MPL) [1, 2]. MPL is a detoxified derivative of lipopolysaccharide from Salmonella minnesota. QS-21-based vaccines induce humoral and Th1-type cellular immune responses [1, 3, 4]. QS-21 has been proposed to act primarily on antigen-presenting cells, enhancing antigen uptake and presentation by dendritic cells. Moreover, it triggers the NLRP3 inflammasome-dependent release of IL-1β and IL-18 [4, 5].
Key features
- Potent vaccine adjuvant
- Strong inducer of antibody-based and cell-mediated immune responses
- Natural and sustainable source
- Chemically and functionally equivalent to bark-extracted QS-21
- Sterile
- Batch-to-batch consistency
- Highly pure (≥ 96%)
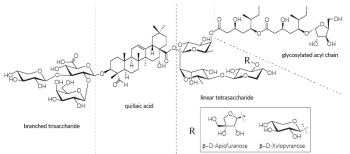
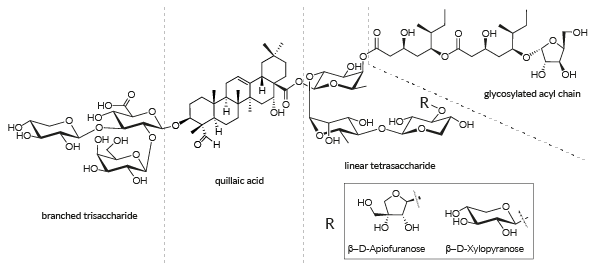
QS-21 is a mixture of two major isomers1,2 with different CAS numbers:
- QS-21 V1 isomer (QS-21-apiose; b-D-Apiofuranose): 141256-04-4-4
- QS-21 V2 isomer (QS-21-xylose; b-D-Xylopyranose): 250643-56-2
QS-21VacciGrade™ is for research use only, and not for human or veterinary use. For your clinical or commercial needs, cGMP cultured plant cell QS-21 with identical biophysical properties is available from SaponiQx Inc.
Note: QS-21 VacciGrade™ is a sterile formulation of SaponiQx's proprietary STIMULON® cultured plant cell QS-21. STIMULON® is a registered trademark Agenus Inc., the parent company of SaponiQx Inc.
AS01® is a registered trademark of GSK.
References:
1. Lv, X., et al., 2024. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 109006.
2. Diderlaurent, A.M. et al., 2017. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16(1):55.
3. Kensil, C.R., 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13:1–55.
4. Lacaille-Dubois, M.A., 2019. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 60:152905.
5. Reinke, S., et al., 2020. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 8(3):554.
All products are for research use only, and not for human or veterinary use.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade, suitable for in vivo studies. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay.
SPECIFICATIONS
Specifications
C92H148O46
1 mg/ml in PBS (pH 6.8) or H2O
< 10 EU/mg (measurement by kinetic chromogenic LAL assay)
Adjuvantation experiments in vivo, in vitro experiments
Vaccine adjuvantation, NLRP3 activation
Each lot is validated using physico-chemical methods.
CONTENTS
Contents
-
Product:QS-21 VacciGrade™
-
Cat code:vac-qs21-1
-
Quantity:1 mg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
The saponin QS-21 isolated from the South American Quillaja Saponaria tree bark is one of the most potent adjuvants and is currently used in several licensed and exploratory human vaccines [1-3]. Notably, STIMULON® QS-21 isolated from bark extract (beQS-21) forms an integral part of the AS01® adjuvant. AS01® is a liposomal mixture of QS-21 and a TLR4 agonist, 3-O-desacyl-4'-monophosphoryl lipid A (MPL) [2, 3]. MPL is a detoxified derivative of lipopolysaccharide from Salmonella minnesota and TLR4 agonist. AS01® containing STIMULON® beQS-21 is a critical component of effective vaccines, including GSK’s Shingrix (Zoster Vaccine Recombinant, Adjuvanted) and Arexvy (Respiratory Syncytial Virus, Adjuvanted) [3]. QS-21 is also a component of Matrix-M adjuvant, which was included in the approved COVID-19 vaccine, NVX-CoV2373, and is used in the R21 malaria vaccine.
QS-21 structure
QS-21 is a mixture of two isomeric molecules, QS-21 β-D-Apiofuranose and QS-21 β-D-Xylopyranose, each having four domains: the triterpene quillaic acid, a branched trisaccharide, a linear tetrasaccharid, and a glycosylated acyl chain [1].
QS-21 adjuvancity
QS-21-based vaccines induce humoral and Th1-type cellular immune responses [4, 5]. Although the modes of action of QS-21 are not fully elucidated, it has been proposed that this triterpene saponin acts primarily on antigen-presenting cells (APCs).
QS-21 is endocytosed by dendritic cells in a cholesterol-dependent manner [6]. The carbohydrate domains and amphiphilic properties of QS-21 are thought to facilitate antigen uptake and presentation by dendritic cells [5].
QS-21, when formulated in liposomes, has been identified as an activator of the NLRP3 inflammasome in antigen-prensenting cells in vitro and in vivo, which results in the release of the potent proinflammatory cytokines IL-1β and IL-18 [7-9]. Importantly, the adjuvant potential of QS-21 via the NLRP3 inflammasome requires additional immunostimulatory substances, such as the TLR4 agonist MPLA [7]. Indeed, TLR priming is a prerequisite for robust inflammasome activation. However, currently available in vivo data for the role of NLRP3 inflammasome in adaptive immune responses warrants further research [7-9].
References:
1. Kensil, C.R., et al., 1991. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146:431.
2. Diderlaurent, A.M. et al., 2017. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16(1):55.
3. Lv, X., et al., 2024. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 109006.
4. Kensil, C.R., 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13:1–55.
5. Lacaille-Dubois, M.A., 2019. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 60:152905.
6. Lorent J.H., et al., 2014. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org Biomol Chem. 12(44):8803-22.
7. Marty-Roix, R., et al., 2016. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem 291:1123.
8. Reinke, S., et al., 2020. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 8(3):554.
9. Detienne, S. et al., 2016. Central role for CD169(+) lymph node resident macrophages in the adjuvancity of the QS-21 component of AS01. Sci Rep. 6:39475.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?