MPLA-SM VacciGrade™
-
Cat.code:
vac-mpla
- Documents
MPLA-SM lots that display a higher potency for human TLR4 are denoted with an asterisk (MPLA-SM* VacciGrade™, cat code vac-mpla2).
ABOUT
Monophosphoryl Lipid A from Salmonella minnesota R595 - Pre-clinical preparation
MPLA-SM VacciGrade™ is a pre-clinical preparation of Monophosphoryl Lipid A (MPLA) for Toll-like receptor 4 (TLR4) activation and vaccine adjuvantation. It is derived from the lipopolysaccharide (LPS, aka endotoxin) of Salmonella minnesota R595 (Re mutant), a rough strain of Gram-negative bacteria.
MPLA-SM is extracted from LPS using treatment with acid and heat followed by chromatography [1]. This preparation contains a mix of MPLA congeneric forms differing in the number of acyl chains. This congener mix is responsible for the partial TLR4 agonist function of some preparations [2]. MPLA-SM is a potent activator of TLR4 and does not activate TLR2 or other TLRs. All lots of MLPA-SM display the same ability to activate murine TLR4 but some lots are more potent than others at inducing human TLR4 responses (see figures).
Note: MPLA-SM lots that display a higher potency for human TLR4 are denoted with an asterisk (MPLA-SM* VacciGrade™, cat code vac-mpla2).
Application
MPLA-SM is a detoxified derivative of lipid A, the biologically active component of LPS. Thus, MPLA-SM features the immunostimulatory effects of LPS without the adverse effects of the native endotoxin [3]. MPLA-SM, as well as other detoxified derivatives of lipid A, including synthetic hexa-acylated MPLA and 3-O-desacyl-4’-monophosphoryl lipid A (also known as MPL), are used as adjuvants in vaccination studies. MPL® is a clinical-grade vaccine adjuvant that is manufactured exclusively by GlaxoSmithKline [1].
MPLA-SM VacciGrade™ is a high-quality pre-clinical grade. It is also available as MPLA-SM in a standard grade for in vitro experiments.
Key features
- Agonist of mouse and human TLR4
- Enhanced activity towards human TLR4 (for MPLA-SM*)
- Strong inducer of Th1 immune response
- Negligible TLR2 activity
- Each lot is functionally tested
References:
1. Wang YQ. et al., 2020. MPL Adjuvant Contains Competitive Antagonists of Human TLR4. Front. Immunol. 11:577823.
2. Kuzmich, NN. et al., 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5unol. 165(2):618-22.
3. Sarkar, I. et al., 2019. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev Vaccines. 18(5):505-521.
All products are for research use only, and not for human or veterinary use.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade, suitable for in vivo studies. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay.
SPECIFICATIONS
Specifications
TLR4
Human, Mouse
2 - 20 μg/mouse
1 mg/ml in DMSO
0.2 µm filtration, Sterility guaranteed
Biological activity has been tested using cellular assays
In vivo experiments
Each lot is functionally tested and validated using cellular assays.
The absence of other bacterial components (e.g. lipoproteins) is controlled using HEK-Blue™ TLR2 cells.
CONTENTS
Contents
-
Product:MPLA-SM VacciGrade™
-
Cat code:vac-mpla
-
Quantity:1 mg
10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
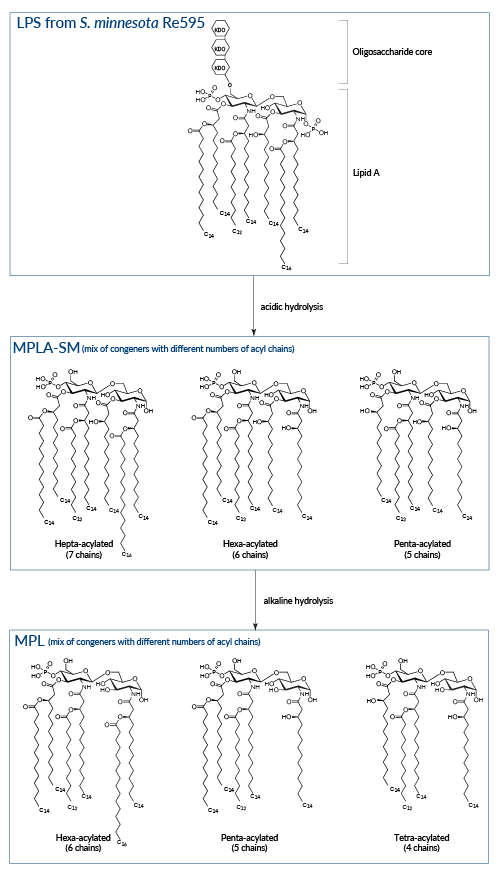
Sequential hydrolysis of LPS from Salmonella minnesota
LPS is a potent activator of TLR4, triggering both NF-κB and IRF-mediated production of pro-inflammatory cyokines and interferons [2]. Thus, LPS features many characteristics needed for an effective vaccine adjuvant. However, large uncontrolled amounts of LPS are extremely toxic and can cause devastating diseases [3]. This led to investigations to define, extract, or synthesize the immunologically active portion of LPS with the lowest toxicity, such as MPLA-SM and MPL®.
LPS
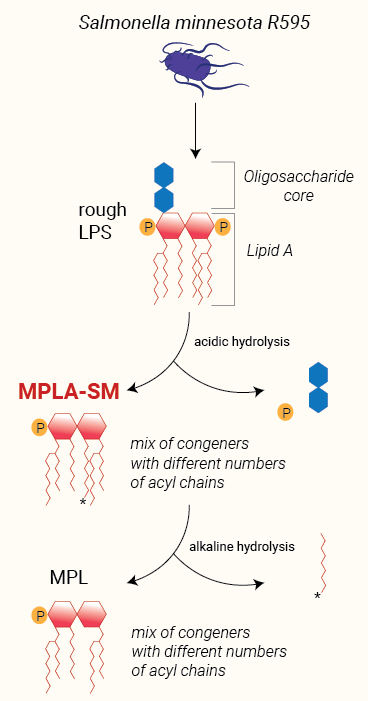
Wild-type LPS, referred to as smooth (sLPS) comprises three covalently linked regions: a Lipid A backbone, a core carbohydrate group, and O-polysaccharide chains. Some bacteria, such as Salmonella minnesota R595, produce a truncated LPS, without O-side chains, referred to as rough (rLPS) [4].
Lipid A
LPS biological activity is mediated by Lipid A recognition by TLR4 and is commensurate to the number of Lipid A fatty acyl chains [3]. Hexa-acylated (6 chains) Lipid A is a highly potent TLR4 agonist, while under‑acylated (4-5 chains) Lipid A induces lower or antagonistic responses [5].
MPLA-SM
The removal of the core carbohydrate group and one phosphate group from the glucosamine disaccharide by acidic treatement of LPS produces MPLA. This derivative displays reduced toxicity while retaining the ability to activate TLR4 [6, 7]. It has been suggested that the reduced toxicity of MPLA is attributed to the preferential triggering of the IRF pathway upon TLR4 activation, resulting in decreased induction of inflammatory cytokines [8]. InvivoGen's MPLA-SM is a research-grade MPLA extracted from LPS of S. minnesota.
MPL
An additional base hydrolysis of MPLA removes one specific fatty acid and generates 3-O-deacylated monophosphoryl lipid A (also known as MPL, 3D-MPL, or 3D-MLA). This deacylation step further decreases the compound residual toxicity. MPL® is a clinical grade vaccine adjuvant that is manufactured exclusively by GlaxoSmithKIine [1].
References:
1. Wang YQ. et al., 2020. MPL Adjuvant Contains Competitive Antagonists of Human TLR4. Front. Immunol. 11:577823.
2. Kuzmich, NN. et al., 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5unol. 165(2):618-22.
3. Steimle, A. et al., 2016. Structure and function: Lipid A modifications in commensals and pathogens. Int J Med Microbiol 306, 290-301.
4. Raetz CR. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59, 129‑70.
5. Cochet, F. & Peri, F. 2017. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18.
6. Qureshi N. et al., 1985. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high-performance liquid chromatography and complete structural determination. J. Biol. Chem. 260, 5271–8.
7. Romero CD. et al., 2011. The Toll-Like Receptor 4 agonist monophosphoryl Lipid A augments innate host resistance to systemic bacterial infection. Infect Immun. 79: 3576–3587.
8. Mata-Haro V. et al., 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 316(5831):1628-32.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?