HEK-Lucia-Star™ ARE Cells
-
Cat.code:
hkls-are
- Documents
ABOUT
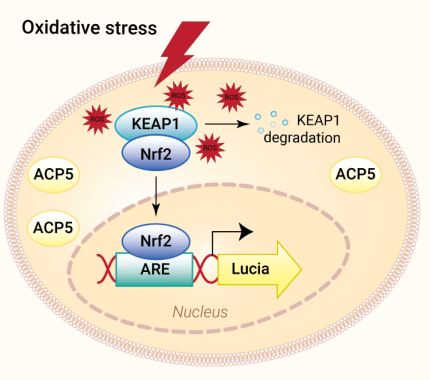
ROS Reporter Cells- Nrf2-KEAP1 pathway
HEK-Lucia-Star™ ARE cells were engineered from the human embryonic kidney HEK 293 cell line. They were designed to monitor the activation of the Nrf2-ARE (antioxidant-responsive element) pathway in response to oxidative stress.
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the ability of cells to scavenge them. High levels of ROS can lead to an apoptosis-like, regulated cell death phenomenon called oxeiptosis. ROS play an important role in the progression of several diseases, including diabetes, atherosclerosis, aging, and cancer [1-2].
Cell line description
The HEK-Lucia-Star™ ARE cell line features the inducible Lucia® luciferase gene under the control of a minimal promoter fused to multimeric antioxidant response elements (ARE). Upon oxidative stress induction, Lucia® luciferase levels can be monitored in the supernatant.
Moreover, these cells constitutively express a secreted version of the tartrate-resistant acid phosphatase 5 (TRAP, ACP5). Measuring the ACP5 level in the supernatant while performing compound screening allows you to assess cell viability simultaneously.
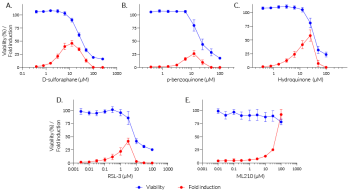
Stimulation with increasing concentrations of oxidant compounds (D-sulforaphane, p-benzoquinone, hydroquinone) or peroxidase inhibitors (RSL-3, ML210) strongly induces oxidative stress responses in HEK-Lucia-Star™ ARE cells (see figure, fold response in red). The loss of the secreted ACP5 signal at higher concentrations of these compounds indicates oxidative stress-induced cell death (see figure, viability percentage in blue). Interestingly no cell death was observed at even the highest concentration of ML210. Treatment with the antioxidant compound N-acetylcysteine decreased the oxidative stress response in HEK-Lucia-Star™ ARE cells in a dose-dependent manner (see figure).
For your convenience, InvivoGen provides easy-to-use liquid detection reagents, QUANTI-Luc™ 4 for Lucia® and QUANTI-Star™ for ACP5, to evaluate the modulatory impact of your compound. Thus, assessing the Lucia® and ACP5 activities provides direct information about the oxidative properties and toxicity effects of your compound of interest.
Features
- Stable expression of two secreted reporter proteins, Lucia® luciferase and ACP5
- Fully functional ARE-dependent Nrf2 KEAP1 pathway
- Readily assessable oxidative stress-inducible Lucia® reporter activity
- Concomitant surveillance of cell viability via ACP5 reporter activity
- Reporter activities measurable in the supernatant (no lysis needed)
Applications
- Studying oxeiptosis and the ARE-dependent pathway via Nrf2 and KEAP1
- Screening of novel ligands with oxidative or antioxidant properties
- Simultaneous monitoring of compound-induced cell death via ACP5 activity
Reference:
1. Hecht F, et al., 2024. Regulation of antioxidants in cancer. Mol Cell.;84(1):23-33.
2. Pichlmair A, et al., 2018. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol.(2):130-140.
These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
Screening of ligands with oxidative or antioxidant properties
Complete DMEM (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Lucia-Star™ ARE Cells
-
Cat code:hkls-are
-
Quantity:3-7 x 10^6 cells
- 1 ml Hygromycin B Gold (100 mg/ml)
- 1 ml Normocin™ (50 mg/ml)
- 1 ml Zeocin® (100 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 0.5 ml of QUANTI-Star™ reagent and 0.5 ml of QUANTI-Star™ buffer (sufficient to prepare 50 ml of QUANTI‑Star™ Solution, a ACP5 detection reagent)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Introduction
Oxidative stress refers to the imbalance between the production of reactive oxygen species (ROS) and the ability of the body to counteract their harmful effects through neutralization by antioxidants [1]. ROS, including free radicals such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, are byproducts of normal cellular metabolism, particularly from mitochondrial oxidative phosphorylation [2]. While ROS play essential roles in cell signaling and homeostasis, excessive amounts can damage cellular components such as lipids, proteins, and DNA, finally leading to oxeiptosis, an apoptosis-like cell death [3]. They are also involved in various pathologies including cancer, neurodegenerative diseases, cardiovascular diseases, and aging [4].
Nrf2-Keap1 pathway
The nuclear factor erythroid 2–related factor 2 (Nrf2) is a critical transcription factor that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation. Nrf2 controls the expression of a set of genes involved in the antioxidant response, detoxification processes, and maintenance of cellular redox homeostasis [2].
Kelch-like ECH-associated protein 1 (KEAP1) is an E3 ligase adaptor protein and the gatekeeper of Nrf2. It serves as a negative regulator of Nrf2. Under normal conditions, Keap1 binds to Nrf2 in the cytoplasm, promoting its ubiquitination and subsequent proteasomal degradation. This tight regulation ensures that Nrf2 levels remain low under homeostatic conditions, preventing unwarranted antioxidant response [2].
Under moderate oxidative or electrophilic stress, the cysteine residues of KEAP1 undergo modification, leading to a conformational change that impairs its ability to target Nrf2 for degradation. This allows Nrf2 to accumulate in the cytoplasm, translocate into the nucleus, and bind to the antioxidant response element (ARE) in the promoter regions of target genes, such as antioxidant enzymes, detoxification enzymes, glutathione, and proteasomal proteins [2].
Oxeiptosis
Interestingly, KEAP1 was found to be a concentration-dependent ROS sensor, that - when the oxidative stress is too high - can mediate oxeiptosis, a recently identified apoptosis-like, caspase-independent, non-inflammatory regulated cell death [3]. This process is Nrf2-independent and characterized by activating KEAP1, phosphoglycerate mutase 5 (PGAM5), and apoptosis-inducing factor mitochondria-associated 1 (AIFM1) - short the KEAP1/PGAM5/AIFM1 pathway [3]. When ROS concentrations are elevated, PGAM5 dissociates from KEAP1 and migrates to the mitochondria. Inside the mitochondria, PGAM5 dephosphorylates the Ser116 residues of AIFM1, finally leading to oxeiptosis; Signaling downstream of AIFM1 which results in cell death is still enigmatic and remains to be elucidated [3].
Nrf2-Keap1 pathway in disease and therapeutics
Due to its crucial role in cellular anti-oxidant defense, the Nrf2-KEAP1 pathway became an attractive therapeutic target in a plethora of diseases [2-5].
In neurodegenerative diseases such as Alzheimer's and Parkinson's, oxidative stress plays a pivotal role in disease progression. Enhancing Nrf2 activity has shown potential in reducing oxidative damage and improving neuronal survival [4].
The role of Nrf2 in cancer is complex and double-edged. While Nrf2 activation protects normal cells from oxidative damage and reduces cancer risk, in established cancers, Nrf2 can promote tumorigenesis and chemoresistance by creating a reductive environment favorable for cancer cell proliferation. Furthermore, the activation of Nrf2 has been proposed as a host-directed therapeutic strategy to treat infectious diseases including COVID-19 [5].
Several therapeutics that aim to modulate the NRF2-KEAP1 pathway are under investigation [4-5]. The first US FDA-approved Nrf2 activator, DMF, has been successfully marketed as a multiple sclerosis (MS) treatment with impressive performance [5]. The modulation of this pathway offers a promising strategy for enhancing cellular defense mechanisms and reducing oxidative stress associated with a wide range of pathological conditions.
References:
1. Hecht F, et al., 2024. Regulation of antioxidants in cancer. Mol Cell.;84(1):23-33.
2. Song MY, et al., 2021. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int J Mol Sci.22(9):4376.
3. Pichlmair A, et al., 2018. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol.(2):130-140.
4. Cuadrado A, et al., 2019. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. (4):295-317.
5. Wang J, et al., 2021. The potential roles of Nrf2/Keap1 signaling in anticancer drug interactions. Curr Res Pharmacol Drug Discov. 2:100028.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?
You may also need
FAQ
HEK293 cell line description
Only our HEK-Blue™ Null1-k and Null2-k cells are sensitive to G418.
The only HEK-Blue™ Null cells that are sensitive to Puromycin are the HEK-Blue™ Null1-v cells.
The only HEK-Blue™ Null cells that are sensitive to Puromycin are the HEK-Blue™ Null1-v cells.
Our RNAseq data confirms that our HEK-Blue™ cells express FcRN, however, this has not been functionally tested.
The difference in activity is approximately 10-fold.
Both cell types overexpress a designated TLR. The primary difference is that HEK-Blue™ cells include the NFκB inducible SEAP reporter construct.
We presume them to be in the endosome as we express the wild-type, full-length genes. Additionally, their signal can be blocked by Chloroquine, an endosomal acidification inhibitor. However, as these TLRs are over-expressed there may potentially be low expression of them on the cell surface.
HEK-Blue™ cells only express a single NFκB inducible SEAP reporter gene. Whereas, HEK-Dual™ cells have the addition of the Lucia™ gene knocked into the IL-8 locus. Thus, when IL-8 is activated following stimulation, HEK-Dual™ cells can report this with the secretion of Lucia™ luciferase.
It should also be noted that the HEK-Dual™ cells have been knocked out for TLR3, TLR5, and TNFR to limit interference from other TLRs when studying a specific TLR pathway.
HEK293 cells express TLR1, TLR3, TLR5, TLR6, and NOD1.
They respond to TLR3, TLR5, and NOD1 agonists, but at a much lower level compared to HEK293 cells transfected with these receptors
HEK-Blue™ IL-1R cells express both human and murine IL-1β receptors, thus can detect both species.
On the other hand, HEK-Blue™ IL-1β cells are specific for human IL-1β, but can still detect higher concentrations of mouse IL-1β.
In the United States, HEK293 cell lines are designated Biosafety Level 2 according to the Center for Disease Control and Prevention (CDC).
In Germany, HEK293 cell lines are designated Biosafety Level 1 according to the Central Committee of Biological Safety, Zentrale Kommission für die Biologische Sicherheit (ZKBS).
You can check with your country’s regulatory authority regarding the use of these cells.
Please note that there is no replicating/infectious Adenovirus 5 in these cells.
The minimal promoter isn't the same but the difference in expression for these two Null cell lines is minor.
There is no specific integration system used to generate our stable cell lines.
The selection pressure is enough to obtain stable clones. The receptors are added by simple transfection of plasmids using a cationic lipidic transfection agent (the plasmids are not linearized before transfection).
Cell line culture
We recommend using a flat-bottom, clear walled cell culture plate.
Below are a few tips we recommend to help get your HEK cells growing:
• For the first 2-3 passages, grow cells in media containing 20% FBS and no antibiotics.
• Do not allow cells to reach 100% confluency Please check cells as regularly as possible.
• The cells should not be grown in 20% FBS for too long. Use media with 10% FBS after 2 or 3 passages.
• When making frozen stocks, continue growing additional cultures in case there is a problem with the frozen stock.
Trypsin does not adversely affect the health or growth of these cells. However, it is known that high concentrations will occasionally induce the activation of NFκB resulting in a higher background in your assay.
Moreover, we have observed some cases where trypsin has been contaminated with TLR2, TLR4, and TLR5 contaminants, which can also interfere with the assay results.
It is not unusual for different TLR cells to grow at different rates. Some TLR clones happen to grow a little slower/faster than others. This is often clone dependent.
When the HEK-Blue™ cells are non-adherent, either they were diluted too harshly at the start or they have grown over-confluent in a small flask and suffocated.
To avoid this in the future:
• Change the media and plate the cells at a density of approximately 1.5 x 106 cells in a T25 flask.
• Wash the cells before putting them into a new flask. Sometimes when the cells are non-adherent, it is due to the clustering of both live and dead cells. Additionally, this will get rid of any remaining DMSO which could affect the adhesion of the cells to the flask.
• Use medium with 20% FBS.
• The use of CellBIND flasks can sometimes help to increase attachment and growth of the cells (however CellBIND flasks are not required in the normal protocol).
The split ratio will depend on when you expect confluency. Typically, the doubling time of HEK-Blue™ cells is approximately 24 hours.
Therefore, if you use a split ratio of 1:2 (50%) into a new flask, cells should be confluent the following day. If you use a split ratio of 1:4 (25%) you can expect the cells to be confluent after 2 days.
Our HEK-Blue™ Selection is provided in 1ml tubes with each containing a 250X solution.
Therefore, you should dilute HEK-Blue™ Selection 1:250 into your media to have a 1X concentration.
HEK-Blue™ cells should be seeded at a density of approximately 1.5 x 106 cells in a T25 flask or 4 – 5 x 106 cells in a T75 flask.
Assays
It depends on the cell line and the concentration of the ligand used to stimulate the cells. In general, we record the results following 16 – 24 hours of stimulation.
There are 2 possible explanations as to why a blue color is observed in all wells.
1. It could be due to the presence of Alkaline Phosphatase (AP) in the culture medium. To see if this is the case, there is a very simple test to perform. Add 50 µl of the medium used for cell culture (without cells) and 200 µl of resuspended HEK-Blue™ detection medium or QUANTI-Blue™. If the medium turns blue, then it is due to the presence of Alkaline Phosphatase (AP) in the serum of the media. In this case, you must heat the serum to inactivate the AP and repeat the medium test. At this point the test should give a negative result (no blue color).
2. It could be due to improper handling of cells before the test. To avoid activation of NFκB before stimulation and reduce the risk of false positive results:
• Use pre-warmed PBS to wash cells
• Use heat-inactivated FBS
• Do not centrifuge cells prior to stimulation
• Do not use trypsin
We have noticed a loss of sensitivity when using HEK-Blue™ Detection medium instead of QUANTI-Blue™ on our cytokine reporter cell lines.
Therefore, we recommend using QUANTI-Blue™, which is provided with the cells, as this is what we use in house.
We recommend to not use any antibiotics at all during assays to ensure the least amount of potential interfering agents in the medium.
Therefore, we do not add HEK-Blue™ Selection to the test media.
We have only tested the use of plasma and serum samples on our HEK-Blue™ hTLR2 cell line.
The results demonstrated that when compared to using standard samples (in DMEM), serum samples give a single log difference.
On the other hand, we found a 3-log difference between DMEM and plasma samples.
This is why we would recommend using serum samples over plasma samples.
Yes, they can be used interchangeably. However, please note that the protocols are distinctly different and need to be followed accordingly.
HEK293 cells are very easy to transfect with a transfection efficiency of approximately 80%.