NexaVant™ VacciGrade™
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
NexaVant™ VacciGrade™ TLR3 agonist - dsRNA-based vaccine adjuvant |
Show product |
100 µg 5 x 100 µg |
vac-nvt
|
|
TLR3 agonist - NexaVant™ (NVT) | Homogeneous and sterile vaccine adjuvant
NexaVant™ VacciGrade™ is a double-stranded (ds) RNA-based TLR3 agonist and potent vaccine adjuvant, provided in a high-quality pre-clinical grade.
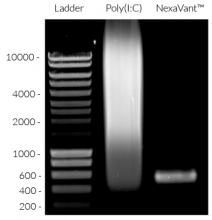
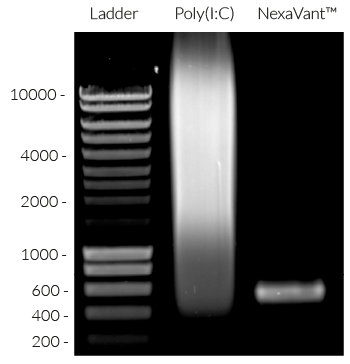
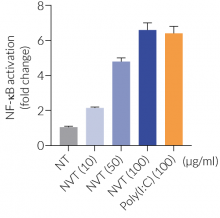
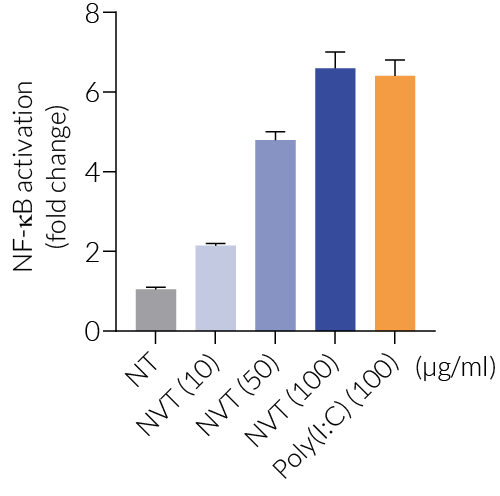
NexaVant™ (NVT) is a synthetic dsRNA of 424 base pairs chosen from the Chinese sacbrood virus (CSBV) genome. It displays high purity, molecular homogeneity, measurable pharmacokinetics, and non-toxicity in various animals, therefore overcoming various obstacles of currently available dsRNA-based adjuvants (e.g. Poly(I:C)) [1]. It is a potent TLR3 agonist verified using HEK-Blue™ hTLR3 cells (see figures) [1]. It also upregulates specific interferon-stimulated genes (ISGs) including RIG-I, MDA-5, and TLR3 [1].
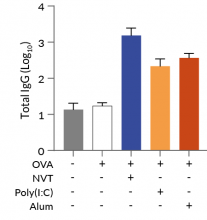
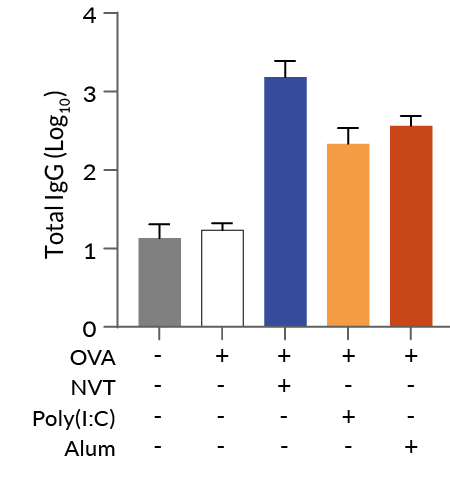
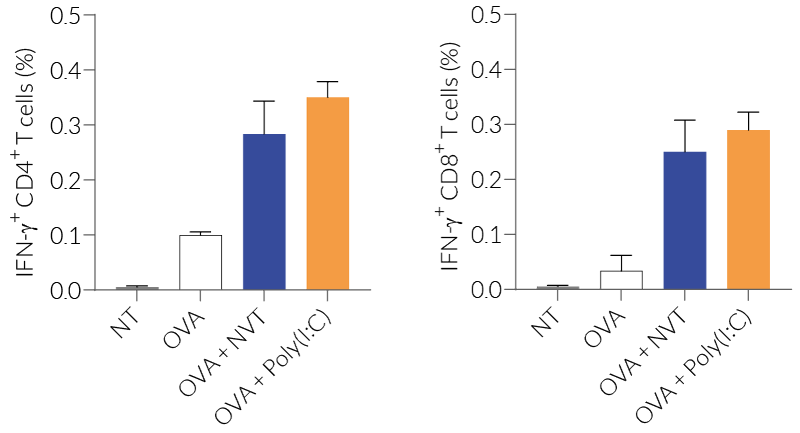
In vivo experiments in mice demonstrated NVT's ability to attract immune cells, activate dendritic cells, and induce strong Th1-type immune responses, such as IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells, surpassing conventional adjuvants like Alum [1]. Moreover, NVT is a promising adjuvant for T cell vaccines, showing enhanced protection against influenza infections when used intranasally, promoting lung-resident memory CD4+ T cells that provide broad and long-term immunity against heterosubtypic strains [2].
InvivoGen also offers NexaVant™ in a standard grade for in vitro experiments.
Production of NVT
A nucleotide segment (1701-2112; 412 nucleotides) from the CSBV genome, which does not match any human DNA sequence, was cloned into a vector. After in vitro transcription using T7 RNA polymerase technology, the remaining DNAs and non-specific ssRNAs were removed with the treatment of DNase I and RNase T1 to generate a dsRNA structure having UAUAG-3′ at both ends. Subsequently, the final product was purified by reverse-phase HPLC [1]. NexaVant™ is available at cGMP grade from the NA Vaccine Institute.
Key features
- Potent TLR3 agonist and vaccine adjuvant
- Strong Th1 responses inducer
- Highly pure (≥ 95%) and stable
- Ready-to-use liquid
- Batch-to-batch consistency
- Each lot is functionally tested
NexaVant™ VacciGrade™ is a high-quality pre-clinical grade. It is also available in a standard grade as NexaVant™.
NexaVant™ is a trademark that belongs to the NA Vaccine Institute.
NexaVant™ VacciGrade™ is for research purposes only; not for human or veterinary use.
References:
1. Ko KH, et al., 2023. A novel defined TLR3 agonist as an effective vaccine adjuvant. Front Immunol. ;14:1075291.
2. Ko KH, et al. 2024. A vaccine platform targeting lung-resident memory CD4+ T-cells provides protection against heterosubtypic influenza infections in mice and ferrets. Nat Commun.15(1):10368.
Specifications
Description: TLR3 agonist VacciGrade™
CAS Number: 2839526-76-8
Polarization of innate immune response: Macrophages, Neutrophils, Dendritic cells
Polarization of adaptive immune response: Th1 response
Quantity: 100 µl (vac-nvt) or 5 x 100 µl (vac-nvt-5)
Concentration: 1 mg/ml
Buffer composition: 10mM Phosphate Buffer pH 7.2
A260/A280 ratio: 1.8 ~ 2.2
Size: 424 bp
Dosing Guidelines:
- Mice: ≥10 µg/dose (as vaccine adjuvant) and ≥ 50 µg/dose (as anti-cancer vaccine)
Quality control:
- Sterility guaranteed
- Each lot is functionally tested using cellular assays
- The absence of bacterial contamination (lipoproteins & endotoxins) has been confirmed using HEK-Blue-Lucia™ hTLR2 and HEK-Blue-Lucia™ mTLR4 cells
- Endotoxin level < 5 EU/mg (measurement by kinetic chromogenic LAL assay)
Contents
NexaVant™ VacciGrade™ is provided as a colorless transparent liquid and is available in two quantities:
- vac-nvt: 100 µg at 1 mg/ml
- vac-nvt-5: 5 x 100 µg at 1 mg/ml
![]() NexaVant™ VacciGrade™ is shipped at room temperature.
NexaVant™ VacciGrade™ is shipped at room temperature.
![]() Upon receipt, store at 4°C. NexaVant™ is stable at RT. However, to avoid contamination, we recommend to keep aliquots at 4°C for short-term storage or -20°C for long-term storage.
Upon receipt, store at 4°C. NexaVant™ is stable at RT. However, to avoid contamination, we recommend to keep aliquots at 4°C for short-term storage or -20°C for long-term storage.
![]() The product is stable for up to 1 year when properly stored.
The product is stable for up to 1 year when properly stored.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
Back to the top
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.