hCTLA4-Fc
-
Cat.code:
fc-hctla4
- Documents
ABOUT
Soluble human CTLA-4 (CD152) fused to an IgG1 Fc domain
Protein description
InvivoGen offers hCTLA4-Fc, a soluble human CTLA-4 (CD152) chimera protein generated by fusing the N-terminal extracellular domain of human CTLA-4 (aa 36-160) to the N-terminus of a human IgG1 Fc domain with a TEV (Tobacco Etch Virus) sequence linker.
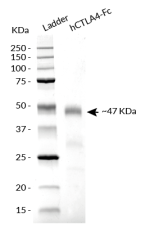
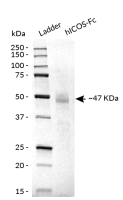
hCTLA-4-Fc has been produced in CHO cells and purified by affinity chromatography. It has an apparent molecular weight of ~47 kDa on an SDS‑PAGE gel.
CTLA-4 background
The cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), also known as cluster of differentiation 152 (CD152) is a Type I transmembrane protein expressed by activated and regulatory T cells. CTLA-4 is an inhibitory receptor and immune checkpoint. It exerts competitive binding to the co-stimulatory receptor CD28 ligands (i.e. CD80 and CD86) expressed by antigen-presenting cells. Thereby CTLA-4 upregulation by T cells prevents overstimulation [1-3].
Applications
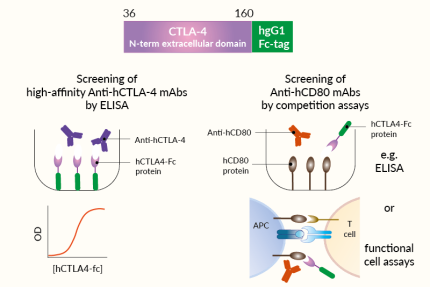
- Screening of high-affinity anti-human CTLA-4 monoclonal antibodies by ELISA
- Screening of anti-human CD80 or CD86 monoclonal antibodies using competition assays
Quality control
- Size and purity confirmed by SDS-PAGE
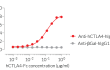
- Protein validated by ELISA using an anti-hCTLA-4 monoclonal antibody
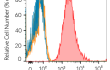
- Protein validated by flow cytometry using Raji-APC-hPD-L1 cells
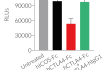
- Protein potency at blocking the CD28/CD80- and CD28/CD86-mediated intracellular signaling validated using Jurkat-Lucia™ TCR-hPD-1 cells
References
1. Wilson, R.A.M. et al. 2018. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin. Exp. Immunol. 191(2):133-148.
2. Wei, S.C. et al. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8(9):1069.
3. Marin-Acevedo J.A. et al. 2018. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11(1):39.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
NP_005250.2
Sodium phosphate buffer with glycine, saccharose and stabilizing agents
ELISA, competition assays, flow cytometry
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:hCTLA4-Fc
-
Cat code:fc-hctla4
-
Quantity:50 µg
1.5 ml of endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
The cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) is an inhibitory receptor and immune checkpoint expressed by activated and regulatory T cells [1, 2].
The current paradigm is that full activation of T cells requires at least 2 signals upon contact with antigen-presenting cells (APCs) [3, 4]. Signal 1 is delivered upon the interaction of the T cell receptor (TCR) with antigenic peptides bound to major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs). Signal 2 is delivered upon the interaction of the co-stimulatory receptor CD28 with the B7 family ligands, B7-1 (CD80) and B7-2 (CD86), on APCs.
Signal 1: TCR and [HLA::peptide]
The 'classical' and most represented TCR is an 80 to 90 kDa heterodimer composed of one α chain and one β chain. The αβTCR is a transmembrane protein expressed by developing and mature T cells. It features an extracellular ligand-binding pocket and a short cytoplasmic tail. Each αβTCR is restricted to a specific complex made of an antigenic peptide and a class I or class II MHC molecule. Human MHC molecules are also known as HLA (human leukocyte antigen). Because of its short cytoplasmic tail, the TCR, once engaged, cannot signal and requires non-covalent association with the CD3 to trigger downstream intracellular signaling and T cell activation [3, 4]. Importantly, signal 1 without co-stimulation results in T cell unresponsiveness or 'anergy', a tolerance mechanism that guards against premature activation.
Signal 2: CD28 and CD80/86
CD28 is a homodimeric and transmembrane protein expressed by T cells. Nearly all human CD4+ T cells and 50% of human CD8+ T cells express CD28. The CD28 interaction with CD80 (aka B7-1) or CD86 (aka B7-2) on APCs, in conjunction with TCR engagement, triggers a co-stimulation signal (signal 2). It results in T-cell proliferation, cytokine production, cell survival, and cellular metabolism [3, 4].
IC signal: CTLA-4 and CD80/86
CTLA-4 exerts competitive binding to the co-stimulatory receptor CD28 ligands (i.e. CD80 and CD86) expressed by antigen-presenting cells. Thereby CTLA-4 upregulation by T cells prevents overstimulation.
Anti-CTLA-4 monoclonal antibodies (mAbs), as well as other immune checkpoints targeting mAbs, are extensively investigated to treat various cancers [2, 5, 6].
References:
1. Ribas A. and Wolchock J.D. 2018. Cancer immunotherapy using checkpoint blockade. Science. 359:1350.
2. Wei, S.C. et al. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8(9):1069.
3. Budd R.C. & Fortner K.A., 2017. Chapter 12 - T Lymphocytes. Kelley and Firestein's Textbook of Rheumatology (Tenth Edition). pages 189-206.
4. Smith-Garvin J.E. et al., 2009. T Cell Activation. Ann. Rev. Immunol. 27:591-619.
5. Wilson, R.A.M. et al. 2018. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin. Exp. Immunol. 191(2):133-148.
6. Marin-Acevedo J.A. et al. 2018. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11(1):39.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?