TNF-α Reporter HEK 293 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
HEK-Blue™ TNF-α Cells Human TNF-α SEAP Reporter Cells |
Show product |
3-7 x 10e6 cells |
hkb-tnfdmyd
|
|
||
|
HEK-Blue™ TNF-α vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
hkb-tnfdmyd-av
|
Notification: Reference #hkb-tnfdmyd-av can only be ordered together with reference #hkb-tnfdmyd.
TNF-α Reporter Cells
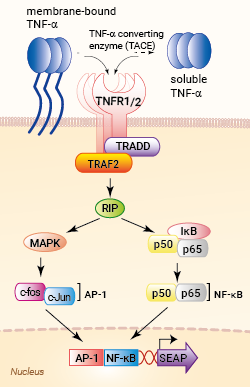
Signaling pathway in HEK-Blue™ TNF-α cells
HEK-Blue™ TNF-α cells were engineered from the human embryonic kidney HEK293 cell line to detect bioactive human and murine tumor necrosis factor-alpha (TNF-α) by monitoring the activation of the NF-κB and AP-1 pathways. In addition, these cells can be used for screening antibodies or small molecule inhibitors targeting the TNF-α pathway.
TNF-α is a multi-functional pro-inflammatory cytokine involved in regulating a wide spectrum of biological processes, such as cell proliferation, differentiation, and apoptosis [1].
Cell line description
HEK-Blue™ TNF-α cells were generated by stable transfection with the genes encoding for the human TNF-α receptor (TNFR1 and TNFR2 chains), as well as an NF-κB/AP-1-inducible SEAP secreted embryonic alkaline phosphatase (SEAP) reporter. The binding of TNF-α to its receptor triggers a signaling cascade leading to NF-κB/AP1 activation and the subsequent production of SEAP. This can be readily assessed in the supernatant using QUANTI-Blue™ Solution, a SEAP detection reagent.
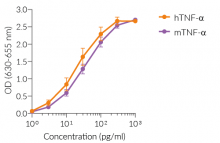
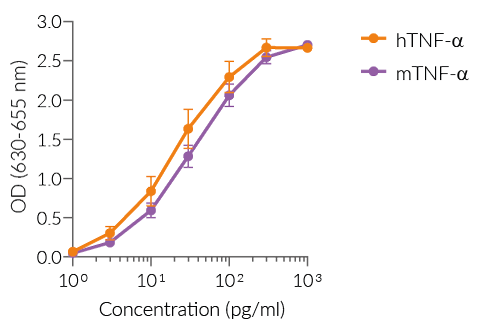
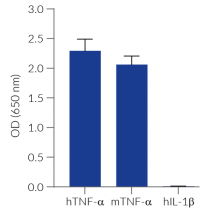
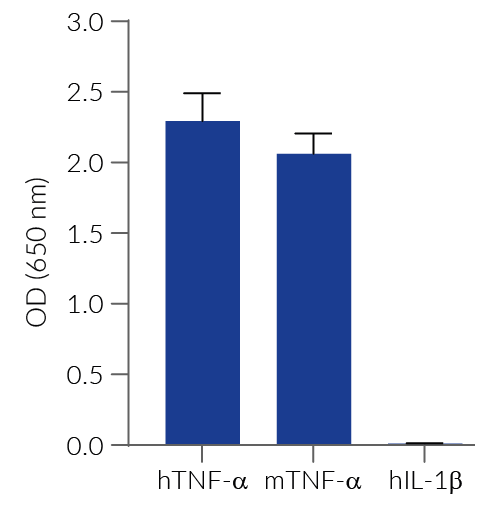
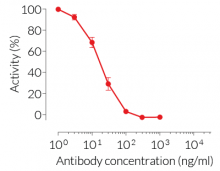
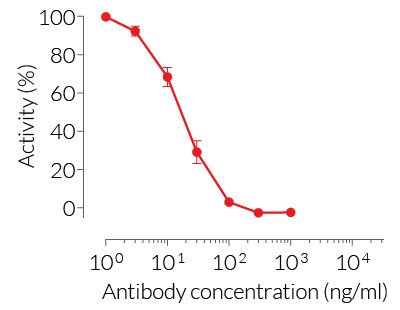
HEK-Blue™ TNF-α cells respond to recombinant human TNF-α, as well as recombinant mouse TNF-α (see figures). Of note, these cells are not responsive to human IL-1β. They can also be used for screening and release assay of molecules that inhibit TNF-α signaling, such as adalimumab, a monoclonal antibody targeting TNF-α.
Key features
- Fully functional TNF-α signaling pathway
- Readily assessable NF-κB/AP-1-inducible SEAP reporter activity
- Strong response to human (h) and murine (m) TNF-α
- No response to hIL-1β
Applications
- Detection and quantification of human and mouse TNF-α activity
- Screening of anti-TNF-α or anti-TNFR antibodies (e.g. adalimumab)
- Screening and release assay of small molecule inhibitors of the TNF-α pathway
Reference:
1. Steeland S, Libert C, Vandenbroucke RE. 2018. A New Venue of TNF Targeting. Int J Mol Sci.;19(5):1442.
Back to the topSpecifications
Antibiotic resistance: Puromycin, Zeocin®
Growth medium: DMEM, 4.5 g/l glucose, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin®
Specificity: Detects human and mouse TNF-α
Detection range:
- hTNF-α EC50: 0.01 ng/ml (in medium) or 0.7 ng/ml (in water)
- mTNF-α EC50: 0.1 ng/ml (in medium) or 3 ng/ml (in water)
Quality Control:
- SEAP reporter activity in response to TNF-α is validated using functional assays.
- The stability for 20 passages following thawing is confirmed.
- These cells are tested for mycoplasma contamination.
Contents
- 1 vial containing 3-7 x 106 cells
- 1 ml of Puromycin (10 mg/ml)
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin® (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Details
Tumor necrosis factor-alpha (TNF-α) is a pleiotropic cytokine involved in necrotic and apoptotic cell death, cellular differentiation, inflammation, and regulation of immune cell activity [1]. Notably, deregulated TNF-α production has been implicated in a variety of conditions, including autoimmune and inflammatory diseases [1].
TNF-α is mainly produced by activated monocytes, macrophages, and T cells. It is first synthesized as a membrane-bound molecule that forms a compact homotrimer through non-covalent interactions. The trimeric membrane-bound form is cleaved by tumor necrosis factor-alpha converting enzyme (TACE) releasing the soluble trimer [2]. Both the membrane-bound and soluble TNF-α bind homotrimeric transmembrane receptors, TNFR1 or TNFR2, triggering signaling pathways that involve TRADD, TRAF2, and RIP, and leading to the activation of NF-κB and MAPK pathways.
1. Steeland S. et al., 2018. A new venue of TNF targeting. Int. J. Mol. Sci. 19:1442.
2. Brenner D. et al., 2015. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 15(6):362-74.