Anti-hTNF-α-hIgG1
-
Cat.code:
htnfa-mab1
- Documents
ABOUT
Anti-human TNF alpha - Adalimumab biosimilar - CAS #331731-18-1
Anti-hTNF-α-hIgG1 is a biosimilar antibody of Adalimumab, a therapeutic antibody that targets tumor necrosis factor-alpha (TNF-α). This monoclonal antibody (mAb) blocks the interaction of soluble and membrane-bound TNF-α with its receptors TNFR1 and TNFR2. Adalimumab is FDA-approved for the treatment of rheumatoid arthritis, Crohn's disease, and psoriasis.
Anti-hTNF-α-hIgG1 comprises the variable region of Adalimumab and the IgG1 constant region of Adalimumab with high effector functions.
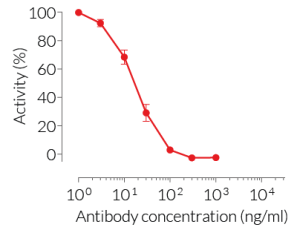
This antibody can be used together with HEK-Blue™ TNF-α cells for screening and neutralization assays to block recombinant human TNF-α-induced signaling (see figure).
Key features
- Each lot is functionally tested and validated
- The complete sequence of the antibody construct has been verified
- Absence of endotoxins determined by the EndotoxDetect assay
More isotypes of this antibody are available and can be used for comparison of biological activities such as ADCC (see in the 'upon request' section).
![]() Read our flyer on Clinically relevant monoclonal antibodies and antibody isotype effector functions.
Read our flyer on Clinically relevant monoclonal antibodies and antibody isotype effector functions.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
TNF-α
Human
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Neutralization assay, ELISA, Fc interaction studies
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hTNF-α-hIgG1
-
Cat code:htnfa-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Tumor necrosis factor-alpha (TNF-α) is a pleiotropic cytokine involved in necrotic and apoptotic cell death, cellular differentiation, inflammation, and regulation of immune cell activity [1]. Notably, deregulated TNF-α production has been implicated in a variety of conditions, including autoimmune and inflammatory diseases [1]. TNF-α is mainly produced by activated monocytes, macrophages, and T cells. It is first synthesized as a membrane-bound molecule (tmTNF) that forms a compact homotrimer through non-covalent interactions. The trimeric membrane-bound form is cleaved by tumor necrosis factor-alpha converting enzyme (TACE) releasing the soluble trimer (sTNF) [2].
Both the membrane-bound and soluble TNF-α bind homotrimeric transmembrane receptors, TNFR1 or TNFR2, triggering signaling pathways that involve TRADD, TRAF2, and RIP, and leading to the activation of NF-κB and MAPK pathways [2]. Importantly, membrane-bound TNF can also mediate a reverse "outside-to-inside" signaling, although this mechanism remains to be fully elucidated [2].
Adalimumab is a therapeutic, fully human IgG1 monoclonal antibody (mAb) that targets and blocks the interaction of both tmTNF-α and sTNF-α with TNFR1 and TNFR2, thereby downregulating the inflammatory responses. Adalimumab is used in the treatment of autoimmune diseases such as rheumatoid arthritis, Crohn's disease, and psoriasis [3].
References:
1. Steeland S., et al. 2018. A new venue of TNF targeting. Int. J. Mol. Sci. 19:1442.
2. Shealy D.J. & Visvanathan S. 2008. Anti-TNF antibodies: lessons from the past, roadmap for the future. Therapeutic Antibodies (book). 101-129.
3. FDA website, visited Dec 2024: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?