diABZI (compound 3)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
diABZI Non-nucleotide-based STING agonist (trihydrochloride form) |
Show product |
2 mg 10 mg |
tlrl-diabzi-2
|
|
Potent non-nucleotide-based STING agonist
diABZI (CAS# 2138299-34-8), also known as diABZI (compound 3) trihydrochloride, is a novel non-nucleotide-based ligand that potently activates STING. Similar to the classical STING agonist, 2'3'-cGAMP, diABZI induces activation of type-I interferons and pro-inflammatory cytokines in vitro and in vivo [1].
diABZI is part of a family of small-molecule amidobenzimidazoles (ABZI) identified to compete with 2'3'-cGAMP [1]. diABZI takes advantage of the symmetrical nature of STING, with two ABZI molecules linked to create a single optimized dimeric ligand [1]. Structural studies have suggested that the activation of STING by classical CDNs requires a closed 'lid' conformation [2]. In contrast, diABZI activates STING while maintaining its open conformation [1]. Further research is needed to understand the implications of this difference.
diABZI represents a novel chemical class with different physicochemical properties (e.g. increased bioavailability) than the classical CDN class of STING agonists. Importantly, this provides more options for the clinical development of STING agonists for the treatment of various conditions (e.g. cancer and viral infection). It has been shown that treatment with diABZI significantly inhibits tumor growth in a mouse model of colorectal cancer [1]. Furthermore, recent papers have highlighted the potential of diABZI in the treatment of SARS-CoV-2 through the induction of an effective IFN response [3-4].
Key Features
- Potent non-CDN STING agonist
- Water-soluble (2 mg/ml)
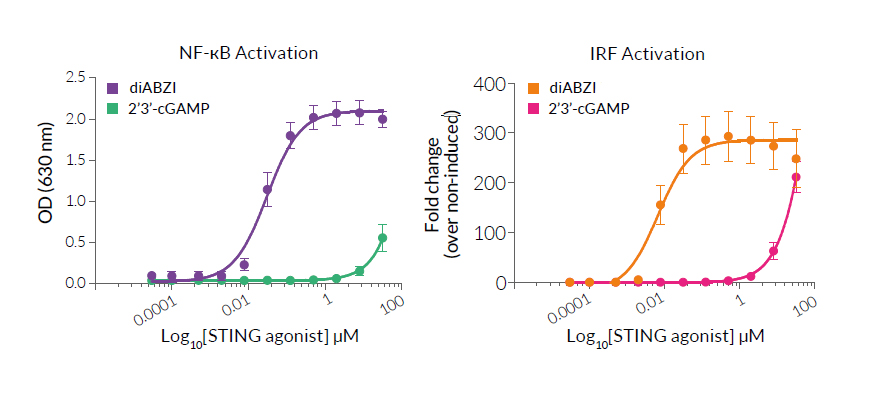
- Activation of the NF-κB- and IRF-dependent responses have been validated with InvivoGen’s THP1‑Dual™ Cells.
References:
1. Ramanjulu, J.M. et al. 2018. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564, 439-443.
2. Shang, G. et al. 2019. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 567(7748), 389–393.
3. Li, M. et al. 2021. Pharmacological activation of STING blocks SARS-CoV-2 infection. Science 6(59), eabi9007.
4. Zhu, Q. et al. 2021. Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system. Antiviral Research 187, 105015.
Specifications
Description: Non-CDN STING agonist
Synonym: diABZI (compound 3)
CAS number: 2138299-34-8
Formula: C42H51N13O7P2 •3HCl
Molecular weight: 959.3 g/mol
Solubility: 2 mg/ml in H2O
Working Concentration: 0.01 - 30 µM
Quality control:
- Purity ≥95% (UHPLC)
- Activation of STING has been confirmed in cellular assays with THP1-Dual™ Cells.
- The absence of endotoxins and other bacterial contaminants has been confirmed using HEK-Blue™ TLR4 and HEK-Blue™ TLR2 cells.
Contents
diABZI is provided as a lyophilized powder and is available in two quantities:
- tlrl-diabzi-2: 2 mg (2 x 1 mg)
- tlrl-diabzi-10: 10 mg (10 x 1 mg)
- endotoxin-free water; 2 x 1.5 ml with tlrl-diabzi-2 and 10 x 1.5 ml with tlrl-diabzi-10
![]() diABZI is shipped at room temperature.
diABZI is shipped at room temperature.
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
Back to the top