Nucleocapsid-His
-
Cat.code:
his-sars2-n
- Documents
ABOUT
SARS-CoV-2 Nucleocapsid with C-term His- or Fc-tag
Protein description
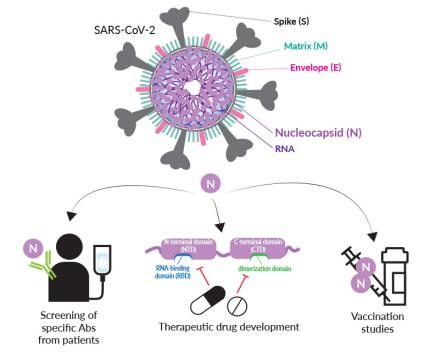
The SARS-CoV-2 (2019-nCoV) Nucleocapsid (N) is a structural protein that plays important roles in the viral life cycle including replication, transcription, and genome packaging [1]. The SARS-CoV-2 N features two important NTD and CTD functional domains [1-6]. NTD interacts with both the RNA genome and Membrane/Matrix (M) proteins to form virion particles. The N protein interaction with the RNA forms the virus ribonucleoprotein core which is packed as a helical “beads-on-a-string” conformation. CTD allows RNA synthesis through binding of the replication-transcription complexes (RTCs), oligomerization of multiple N proteins through its dimerization domain, and genome incorporation into the new virion.
N is a major immunogen of SARS-CoV-2. Indeed, elevated Anti-SARS-CoV-2 N IgG and IgM antibody titers have been reported in COVID-19 patients’ sera [7-9]. These observations make SARS-CoV-2 N an attractive tool for early diagnosis [7-9], and treatment strategies [3].
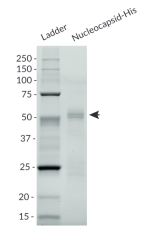
Nucleocapsid-His and Nucleocapsid-Fc were generated by fusing the full-length SARS-CoV-2 N [M1-A419] to a C-terminal poly-histidine sequence and human IgG1 Fc region, respectively. Of note, the SARS-CoV-2 viral sequence used is from the Wuhan-Hu-1 (D614) isolate.
Nucleocapsid-His and Nucleocapsid-Fc have been produced in CHO cells and HEK293 cells, respectively, and have been purified by affinity chromatography (See Specifications for more information).
Applications
- Vaccination studies: using combinations of Nucleocapsid protein antigens and adjuvants
- Antibody screening: finding anti-Nucleocapsid antibodies in COVID-19 patients' sera
- Inhibitor screening: finding small molecules able to block the SARS-CoV-2 Nucleocapsid interaction with replication-transcription complexes (RTCs)
Quality control
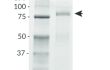
- Size and purity confirmed by SDS-PAGE
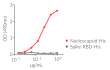
- Protein validated by ELISA using a coated anti-SARS Nucleocapsid antibody
![]() Learn more about SARS-CoV-2 infection cycle, immune responses, and potential therapeutics.
Learn more about SARS-CoV-2 infection cycle, immune responses, and potential therapeutics.
References
1. Mu, J. et al., 2020. SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Sci China Life Sci 63, 1-4.
2. Chang C. et al., 2006. Modular organization of SARS coronavirus nucleocapsid protein. J. Biom. Sci. 13:59-72.
3. Krokhin O. et al., 2003. Mass spectrometric characterization of proteins from the SARS virus. Mol. & Cell. Prot. 2:346-356.
4. Cubuk, J. et al., 2020. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. bioRxiv. doi:10.1101/2020.06.17.158121.
5. Kang, S.et al., 2020. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm Sin B. doi:10.1016/j.apsb.2020.04.009.
6. Khan, M.T. et al., 2020. SARS-CoV-2 nucleocapsid and Nsp3 binding: an in silico study. Arch Microbiol. doi: 10.1007/s00203-020-01998-6.
7. Liu, W. et al., 2020. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J Clin Microbiol 58.
8. Guo L. et al., 2020. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clinical Infectious Diseases. 71(15) :778-785.
9. To K. K-W. et al., 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet Infectious Diseases. 20(5):565-574.
All products are for research use only, and not for human or veterinary use.
InvivoGen also offers:
SPECIFICATIONS
Specifications
P0DTC9
Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
ELISA
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Nucleocapsid-His
-
Cat code:his-sars2-n
-
Quantity:50 µg
1.5 ml of endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Plasmid Sequence
Safety Data Sheet
Certificate of analysis
Need a CoA ?