Anti-PD-L1 (Atezolizumab biosimilar - IgG1 (N298A) isotype)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hPD-L1-hIgG1 (N298A) Human PD-L1 (Atezolizumab) antibody - Human IgG1 (N298A) |
Show product |
100 µg 1 mg |
hpdl1-mab12
|
|
Human IgG4 (N298A) engineered monoclonal antibody (mAb) against human PD-L1

Principle of PD-1/PD-L1 cellular assay
(click to enlarge)
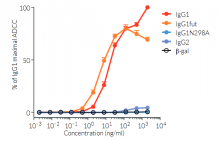
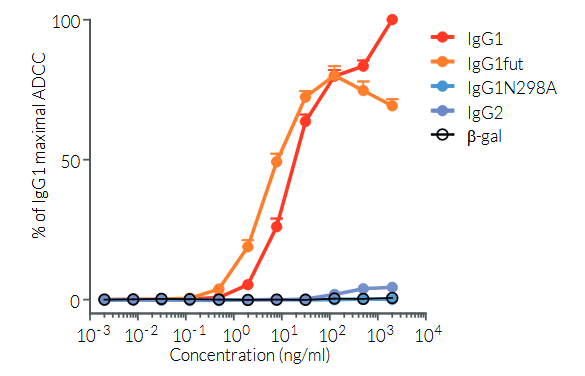
Anti-hPD-L1-hIgG1 (N298A) is the biosimilar of the clinical antibody atezolizumab. Anti-hPD-L1-hIgG1 (N298A) is a recombinant monoclonal antibody (mAb) featuring a variable region of atezolizumab, targeting human (h)PD-L1, and a constant region of the engineered hIgG1 (N298A) isotype. This isotype is designed to limit antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), by eliminating the ability of the mAb to bind to human Fcγ receptors. Anti-hPD-L1-hIgG1 (N298A) was generated by recombinant DNA technology, has been produced in Chinese hamster ovary (CHO) cells, and purified by affinity chromatography with protein G.
PD-L1 background
Programmed cell death ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a transmembrane protein that can be constitutively expressed or induced in myeloid, lymphoid, and normal epithelial cells, as well as in cancer [1, 2]. PD-L1 is the principal ligand for programmed cell death protein 1 (PD-1) and under physiological conditions, this interaction is essential in the development of immune tolerance preventing excessive immune cell activity. However, PD-L1 expression is an immune evasion mechanism exploited by various malignancies and is generally associated with poorer prognosis [3]. Specifically, over-expressed PD-L1 on tumor cells and tumor-infiltrating immune cells, such as macrophages, can bind to PD-1 on cytotoxic T cells, and ultimately inhibit the anti-tumor T cell response [2, 4]. Thus, due to PD-L1’s instrumental role in immune evasion by cancer cells, there are numerous inhibitors in development as promising immuno-oncology therapies. Notably, Atezolizumab (also known as MPDL3280A), a fully humanized IgG1 (N298A) mAb that blocks the interaction of PD-L1 with PD-1 and induces anti-tumor immune reactivation, has been approved by the FDA for combinational use in the treatment of lung and breast cancer [2, 5].
Key features of Anti-hPD-L1-hIgG1 (N298A):
- Clinically relevant variable region targeting PD-L1
- Features the engineered hIgG1 (N298A) constant region for limited effector function
- Functionally validated by flow cytometry
The terms “Atezolizumab” and “MPDL3280A” are only used as references. Anti-hPD-L1-hIgG1 (N298A) is not a pharmaceutical biosimilar of Atezolizumab. It has not been developed nor approved by Atezolizumab owner(s), and is not intended for any therapeutic or diagnostic use in humans or animals.
References
1. Juneja, V.R. et al. 2017. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214, 895-904.
2. Kythreotou, A. et al. 2018. PD-L1. J Clin Pathol 71, 189-194.
3. Sun, C. et al. 2018. Regulation and Function of the PD-L1 Checkpoint. Immunity 48, 434-452.
4. Lau, J. et al. 2017. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8, 14572.
5. Heimes, A.S. & Schmidt, M. 2019. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs 28, 1-5.
Specifications
Specificity: Targets cells expressing human or murine PD-L1
Clonality: Monoclonal antibody
Clone: Atezolizumab (anti-hPD-L1-hIgG1 (N298A), kappa)
Isotype: Human IgG1 (N298A), kappa
Control: Human IgG1 (N298A)
Source: CHO cells
Formulation: 0.2 µm filtered solution in 68 mM sodium phosphate buffer (pH 7.4) with 91 mM glycine, 5% w/v saccharose and stabilizing agents
Purification: Purified by affinity chromatography with protein G
Tested applications: Flow cytometry, ADCC, Disruption of PD-1/PD-L1 inhibitory interaction
Quality Control:
- Binding of Anti-hPD-L1-hIgG1 (N298A) to human PD-L1 has been validated using flow cytometry.
- The complete sequence of this antibody has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Contents
Anti-hPD-L1-hIgG1 (N298A) purified monoclonal antibody is provided azide-free and lyophilized. It is available in two quantities:
- hpdl1-mab12: 100 µg
- hpdl1-mab12-1: 1 mg
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
Tags: buy Atezolizumab (anti-PD-L1) | Atezolizumab (anti-PD-L1) supplier | purchase Atezolizumab (anti-PD-L1) | Atezolizumab (anti-PD-L1) cost | Atezolizumab (anti-PD-L1) manufacturer | order Atezolizumab (anti-PD-L1) | Atezolizumab (anti-PD-L1) distributor
Back to the top