Repurposing approved drugs to target SARS-CoV-2
Current treatments
As of April 2020, there are no specific therapies approved for the treatment of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). One of the strategies for the treatment of COVID-19 is to repurpose approved drugs, known to act on different stages of both the infection and host response. The use of these drugs fast-tracks a treatment plan for COVID-19 as they have known favorable safety profiles for patients.
Treatment targets in SARS-CoV-2 infection
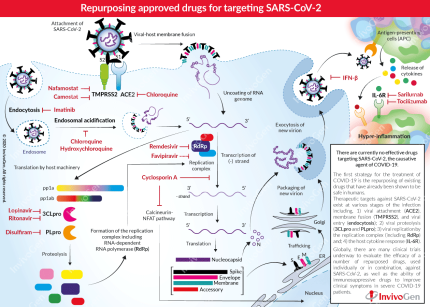
Therapeutic targets against COVID-19 exist at various stages of the infection and disease progression:
- Viral attachment and entry
SARS-CoV-2 binds to two host surface-expressed proteins, ACE2 and the serine protease, TMPRSS2, through its Spike (S) protein. The viral S protein is cleaved into two functional subunits, S1 which interacts with ACE2, and S2 that is further cleaved and activated by TMPRSS2. Together, these actions result in viral-host membrane fusion [1]. These high-affinity interactions are essential in viral entry and are therefore prime targets in the treatment of COVID-19. Chloroquine has formerly been shown to interfere with the terminal glycosylation of ACE2, and thus negatively influences the virus-receptor binding in SARS-CoV infection [2]. Additionally, Camostat and Nafamostat are both clinically proven inhibitors of TMPRSS2 that have shown effectiveness against coronaviruses (e.g. MERS-CoV) [1, 3].
The β-coronaviruses, SARS-CoV and MERS-CoV, have been shown to enter the host cell by endocytosis [4]. During endocytosis, the virion is surrounded by the cell membrane and internalized via the formation of cytoplasmic vesicles termed 'endosomes'. Subsequent endosomal acidification is required to obtain efficient host cell infection. The role of endocytosis is not yet elucidated in SARS-Cov-2 infection [5]. Nevertheless, it is a possible target through the repurposing of Imatinib, an Abelson (Abl) kinase inhibitor, that has been shown to block endocytic entry of other β-coronaviruses [6]. Furthermore, endosomal acidification may be inhibited with Chloroquine [2] or its more potent chemical derivate Hydroxychloroquine [7, 8], which have shown promise in testing with SARS-CoV-2 in vitro.
- Viral proteolysis
Upon the release of viral RNA into the host cytoplasm, the host machinery is used to translate the essential viral polyproteins (pp1a and pp1ab), which include the proteases 3CLpro and PLpro. These viral proteases are responsible for the proteolysis of the polyproteins into effector proteins [4]. 3CLpro is an important protease for a number of viruses such as HIV and thus has been previously targeted for drug development. The fixed-dose combination of Lopinavir & Ritonavir (sold under the name Kaletra), is known to inhibit the activity of 3CLpro and is approved for the treatment of HIV/AIDS [9]. Preliminary clinical trials using Kaletra for the treatment of SARS-CoV-2 have been disappointing [10], however, it is currently being evaluated in combination with other antiviral drugs. Additionally, a clinically available alcohol-aversive drug, Disulfiram, has been shown to inhibit PLpro in both MERS-CoV and SARS-CoV [11].
- Viral replication
Viral replication requires the ‘replication-transcription complex’ consisting of a number of components including the viral RNA-Dependent RNA polymerase (RdRp) and helicase [4]. It has been reported that the most promising antiviral agent against COVID-19 is Remdesivir, an adenosine analog, developed to combat other viruses (e.g. Ebola virus) [12]. It has proven to be highly effective against SARS-CoV-2 in vitro, with its active form able to incorporate into nascent viral RNA by RdRp and ultimately, causing RNA synthesis arrest [13, 14]. Additionally, the antiviral Favipiravir, approved in China for the treatment of Influenza, is recognized as a substrate by viral RdRp and thereby inhibits its activity. It has also been shown to be effective against a number of viruses [9].
Cyclosporin A is an approved immunosuppressant drug for a number of conditions such as Crohn’s disease. Importantly, Cyclosporin A has shown effectiveness against a wide range of viruses in vitro, including coronaviruses, by interfering with protein interactions and thereby affecting viral replication [15]. Therefore, its effect in the treatment of COVID-19 may be two-fold, against both the virus and the hyper-inflammation response.
- Host cytokine response
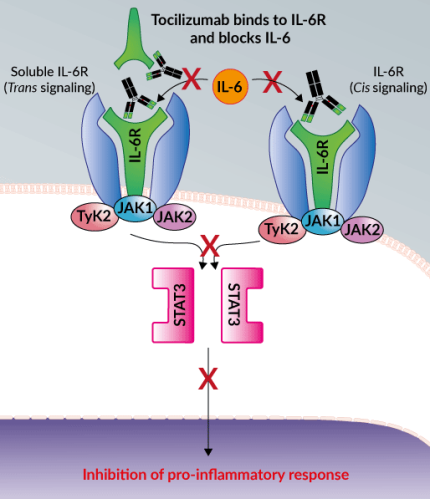
A coordinated, controlled, and balanced cytokine response is essential for the host immune response to SARS-CoV-2. However, severe cases of COVID-19 show a sustained decrease in CD4 and CD8 T cells and an increase in inflammatory cytokines including IL-6 which can cause the cytokine storm. Blocking the signal transduction pathway of IL-6 using monoclonal antibodies (mAbs) against the IL-6 receptor (IL-6R), such as Tocilizumab (TCZ) and Sarilumab, is emerging as a promising strategy to prevent the cytokine storm [18]. In particular, TCZ binds to both soluble and cell-associated IL-6R with high affinity. TCZ blocks IL-6 from initiating its pro-inflammatory downstream signaling, alleviating the consequential immune responses [19]. TCZ has previously been approved by the FDA for the treatment of inflammatory diseases such as rheumatoid arthritis, as well as the reversal of cytokine release syndrome, a form of “cytokine storm” [19]. Additionally, preliminary in vitro data suggest that SARS-CoV-2 is more susceptible to type I interferon (e.g. Interferon-β) treatment than SARS-CoV [20].
- Other lines of research
Scientists are also exploring other strategies to develop safe and effective COVID-19 therapeutic molecules. These include the screening of large chemical libraries and the development of novel molecules based on artificial intelligence (AI) simulations of SARS-Cov-2 and its interaction with the host. Besides small molecules, antibodies represent a very promising approach for the treatment of COVID-19. Antibodies derived from immunized individuals that target the virus specifically (e.g. the Spike protein) are currently under investigation and/or in clinical trials.
References
1. Hoffmann, M. et al. 2020. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell
2. Vincent, M.J. et al. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2, 69.
3. Yamamoto, M. et al. 2016. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob Agents Chemother 60, 6532-6539.
4. Fehr, A.R. & Perlman, S. 2015. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282, 1-23.
5. Guo, Y.R. et al. 2020. The origin, transmission, and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 7, 11.
6. Sisk, J.M. et al. 2018. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol 99, 619-630.
7. Liu, J. et al. 2020. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6, 16.
8. Zhou, D. et al. 2020. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother
9. Dong, L. et al. 2020. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 14, 58-60.
10. Cao, B. et al. 2020. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N Engl J Med
11. Lin, M.H. et al. 2018. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res 150, 155-163.
12. Martinez, M.A. 2020. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother
13. de Wit, E. et al. 2020. Prophylactic and therapeutic Remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 117, 6771-6776.
14. Wang, M. et al. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269-271.
15. de Wilde, A.H. et al. 2011. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 92, 2542-2548.
16. Huang, C. et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497-506.
17. Li, H. et al. 2020. Updated approaches against SARS-CoV-2. Antimicrob Agents Chemother
18. Luo, P. et al. 2020 Tocilizumab treatment in COVID‐19: a single-center experience. J Med Virol.
19. Zhang, S. et al. 2020. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Investig
20. Lokugamage, K.G. et al. 2020. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv.
May 11, 2020 - Update to the clinical status of repurposed drugs for the treatment of COVID-19
Ongoing clinical trials
There are currently over 1200 clinical trials registered on clinicaltrials.gov investigating the possible treatments for COVID-19. These trials are still in the early stages, and therefore, any results need to be taken with extreme caution. Additionally, there are a number of study limitations such as study design, cohort demographics, and cohort size, that need to be considered, especially when conflicting data is observed.
As clinical testing is ongoing, the data is constantly growing and changing. Furthermore, many of the results are preemptively being announced by the media, prior to submission to peer-reviewed journals. In some cases, this is painting an incomplete and biased picture of the data and as such, should be treated carefully. During this global pandemic, where science is racing to find an effective treatment for COVID-19, it is important to analyze the data accurately before drawing any conclusions.
Update on Hydroxychloroquine clinical trials
Chloroquine (CQ) and its less toxic derivative, Hydroxychloroquine (HCQ), are long-standing, FDA-approved anti-malarial and anti-rheumatic drugs, respectively. Importantly, both have been shown to have antiviral activity against SARS-CoV-2 in vitro and immunomodulatory capacities that may be useful in the treatment of COVID-19 [1]. However, HCQ was reported to be more potent against SARS-CoV-2 in vitro [2]. Therefore, with the known dose-dependent toxicity profile of HCQ in humans, compared to that of CQ, HCQ offers a cheap and safer treatment option for COVID-19.
Early human ‘discovery’ trials (single-center, non-randomized, small cohorts) carried out in France and China showed that HCQ was efficient in clearing viral nasopharyngeal carriage of SARS-CoV-2 in COVID-19 patients after approximately 6 days [3]. Furthermore, it was noted that there was more efficient viral clearance using the combination of HCQ and the antibiotic, Azithromycin (AZ) [3]. The combination of CQ and AZ has previously been studied in numerous safety trials involving malaria patients [4]. However, in the context of COVID-19, Azithromycin was originally used to prevent any opportunistic bacterial respiratory infections in patients [3]. Furthermore, it has also been shown in vitro to have anti-inflammatory and importantly, anti-viral effects [5]. Following the observed additive benefit of HCQ and AZ for COVID-19 treatment, modeling and in vitro studies proposed a pharmacological rationale for using this combination in patients. Their apparent synergistic effect may possibly be due to each drug disrupting the interaction between the viral spike (S) protein and the host ACE2 receptor (in differing ways) [6,7]. Also, it is known that there is “a tight window” for both drugs to reach high enough levels so that they have antiviral activity without causing toxic side effects, such as severe gastrointestinal symptoms and cardiac toxicity [4].
Despite early endorsement and ongoing support of HCQ by global leaders, to date, the reported ‘evidence’ for its use in COVID-19 treatment is based solely on non-controlled studies. Therefore, these preliminary results need to be confirmed or disapproved with larger cohort studies. Currently (as of the 6th May 2020), 171 trials for COVID-19 involving HCQ are registered on clinicaltrial.gov, with an average cohort of 1400 patients. Among those, there are 55 trials aiming specifically to evaluate the combination of HCQ and AZ. Furthermore, other drugs such as Anti-IL-6R mAbs (Tocilizumab and Sarilumab) and the HIV drug combination, Lopinavir / Ritonavir, are being tested in combination with HCQ alone or with AZ.
Recently, several reports have cast doubts over the effectiveness of HCQ for the treatment of COVID-19, with no clinical difference observed in patients when used alone or in combination with AZ [8,9]. Additionally, serious safety concerns have been reported in a recent trial where higher doses of CQ alone were associated with an increased incidence of death in COVID-19 patients [10]. There is still no definitive evidence for the repurposing of HCQ to treat COVID-19 patients. Therefore, the ongoing, larger, randomized, controlled clinical studies will ultimately, and hopefully conclusively, answer the question of whether HCQ alone or in combination is able to effectively, and safely, treat COVID-19.
Update on Remdesivir clinical trials
Remdesivir, also known as GS-5734, is an adenosine analog with broad-spectrum antiviral activity, developed by Gilead for the treatment of Ebola [11]. It has potent activity against an array of RNA viruses through the targeting of the viral RNA dependent RNA polymerase (RdRp).
Despite not being approved for Ebola treatment, it has been demonstrated in vivo to be effective in treating both severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [12,13]. Importantly, early in the COVID-19 pandemic, remdesivir was reported to inhibit SARS-CoV-2 infection efficiency in vitro [14]. Additionally, it has since been found that early administration of remdesivir during SARS-CoV-2 infection reduces the viral load and damage to the lungs in an infected rhesus macaque model [15].
Clinical improvements (e.g. need for oxygen support) have been observed in small cohorts of severe COVID-19 cases treated with remdesivir [16]. However, these early studies have a number of limitations including, small cohort sizes and uncontrolled data collection (no control/placebo group). Since then, a number of clinical trials have been launched around the world to test the drug's safety and efficacy. Recently published in The Lancet, an initial randomized, double-blind, placebo-controlled clinical trial (i.e. ‘gold-standard’ clinical trial) conducted in China with 237 patients, found that remdesivir was not associated with statistically significant clinical benefits in the treatment of severe COVID-19. However, a reduction in time to clinical improvement, in those patients treated earlier in the disease, was noted [17]. In support of this observation, it has been recently announced that in a larger (> 1000 patients), global (from 68 countries), ‘gold-standard’ clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), remdesivir was able to meet its primary endpoint, time to recovery. The director of NIAID, Dr. Anthony Fauci, officially announced that remdesivir was able to reduce the recovery time for hospitalized COVID-19 patients from 15 to 11 days (or 31%), providing proof of concept that a drug can block the virus. It was also noted in the media that in addition to recovering more quickly, on average, fewer people died in the remdesivir treated group (~8%) compared to the placebo group (~11%), though, this result wasn't found to be statistically significant. Remdesivir has now been approved for emergency use by the FDA and is a “new standard of care” for all other trials currently taking place. Presently (as of the 6th May 2020), there are 21 clinical trials involving remdesivir registered on clinicaltrials.gov for the treatment of COVID-19, including an NIH clinical trial testing remdesivir in combination with the anti-inflammatory drug (JAK1/2 inhibitor) baricitinib.
Update on Tocilizumab (Anti-IL-6R) clinical trials
Severe COVID-19 patients have been shown to exhibit high levels of pro-inflammatory cytokines, such as IL-6, in their plasma, which can ultimately initiate life-threatening hyper-inflammation and a “cytokine storm” in the lungs and other organs [18, 19]. Therefore, Tocilizumab (TCZ), a humanized anti-human interleukin-6 receptor (IL-6R) monoclonal antibody (mAb) developed by Genentech, is being explored as a potential therapeutic in the treatment of these patients [20].
A small cohort (21 patients), retrospective study in China has reported that TCZ can almost immediately improve clinical outcomes in severe COVID-19 patients. It was observed that within 1-day patient fevers were returning to normal, and within 5 days, 75% of the patients had lowered their need for oxygen [21]. Additionally, after 5 days 85% of patients were found to have a lower percentage of IL-6 producing cells, an important indicator for diagnosis and disease severity in COVID-19 patients [21]. Importantly, no adverse side effects of TCZ were noted in this limited study. Recently, in support of these preliminary results, it has been reported in the media that an ongoing French study involving 129 patients has observed that TCZ "significantly" reduced the number of deaths or life support interventions in COVID-19 patients compared with the control group.
Currently (as of the 6th May 2020), 36 trials for COVID-19 involving TCZ are registered on clinicaltrials.gov. TCZ appears to be a promising approach in the management of severe COVID-19 disease, and the larger, ‘gold standard’ clinical trials currently underway will hopefully confirm these results in the near future.
References
1. Liu, J. et al. 2020. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6, 16.
2. Yao, X. et al. 2020. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis
3. Gautret, P. et al. 2020. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents, 105949.
4. Damle, B. et al. 2020. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther
5. Menzel, M. et al. 2016. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci Rep 6, 28698.
6. Andreani, J. et al. 2020. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows a synergistic effect. Microb Pathog 145, 104228.
7. Sandeep, S. & McGregor, K. 2020. Energetics Based Modeling of Hydroxychloroquine and Azithromycin Binding to the SARS-CoV-2 Spike (S)Protein - ACE2 Complex. ChemRxiv.
8. Magagnoli, J. et al. 2020. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv
9. Molina, J.M. et al. 2020. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect
10. Borba, M.G.S. et al. 2020. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open 3, e208857.
11. Mulangu, S. et al. 2019. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med 381, 2293-2303.
12. de Wit, E. et al. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 117, 6771-6776.
13. Sheahan, T.P. et al. 2017. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9
14. Wang, M. et al. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269-271.
15. Williamson, B.N. et al. 2020. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv
16. Grein, J. et al. 2020. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med
17. Wang, Y. et al. 2020. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. The Lancet
18. Chen, G. et al. 2020. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest
19. Huang, C. et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497-506.
20. Zhang, S. et al. 2020. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Investig
21. Xu, X. et al. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A