The cytosolic NOD-Like Receptors (NLRs, also known as CATERPILLERs, NODs or NALP/PAN/PYPAFs) are nucleotide-binding oligomerization domain containing receptors.
To date, 22 NLRs have been identified in humans and constitute a major class of intracellular pattern recognition receptors (PRRs).
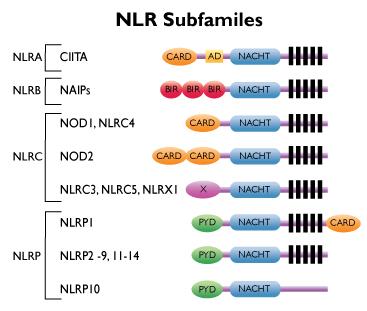
The mammalian NLRs can be divided into four subfamilies, based on different N-terminal effector domains. The effector domains found in NLRs are CARDs, pyrin domains (PYDs), baculoviral inhibitor of apoptosis repeat (BIR) domains, or the transactivator domain (AD).
The designated subfamilies are (based on the initial of the domain name): NLRC (formely known as NODs), NLRP (formerly known as NALPs), NLRB (formely known as NAIP or Birc) and NLRA.
NLRs sense infection and stress through the recognition of cytoplasmic pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), respectively.
Subsequently, NLRs orchestrate an inflammatory response, autophagy or cell death.
The physiological importance of these cytosolic sensors is underscored by a high incidence of genetic mutations that are associated with chronic inflammatory or autoimmune disorders.
NOD1 and NOD2
The founding NLRs members NOD1 (CARD4) and NOD2 (CARD15) fall into the NLRC subfamily and contain one and two N-terminal CARD domains, respectively.
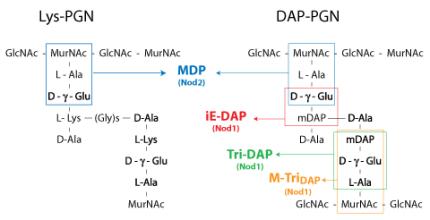
NOD1 and NOD2 recognize distinct motifs of peptidoglycan (PGN), an essential constituent of the bacterial cell wall.
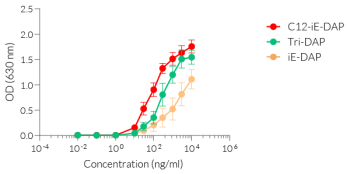
NOD1 senses the D-γ-glutamyl-meso-DAP dipeptide (iE-DAP), which is found in PGN of all Gram-negative and certain Gram-positive bacteria [1, 2] whereas NOD2 recognizes the muramyl dipeptide (MDP) structure found in almost all bacteria.
Thus NOD2 acts as a general sensor of PGN and NOD1 is involved in the recognition of a specific subset of bacteria.
Both iE-DAP and MDP must be delivered intracellularly either by bacteria that invade the cell or through other cellular uptake mechanisms. Ligand bound NOD1 and NOD2 oligomerize and signal via the serine/threonine RIP2 (RICK,CARDIAK) kinase through CARD-CARD homophilic interactions [3].
Once activated, RIP2 mediates ubiquitination of NEMO/IKKγ leading to the activation of NF-κB and the production of inflammatory cytokines. Furthermore, poly-ubiquitinated RIP2 recruits TAK1, which leads to IKK complex activation and the activation of MAPKs [4]. Signaling by NOD2 has been shown to involve the adapter protein CARD9, to mediate p38 and JNK signaling through RIP2 [5].
Genetic mutations in NOD2 are associated with Crohn’s disease, a chronic inflammatory bowel disease [6].
NLRC4 and NLRX1
NLRC4 (IPAF, CLAN/CARD12) belongs to the NLRC subfamily. NLRC4 plays a key role in the regulation of caspase-1 by forming a multiprotein complex called the “inflammasome”.
Caspase-1 participates in the processing and subsequent release of proinflammatory cytokines, such as IL-1β and IL-18. Caspase-1 activation induced by cytosolic flagellin has been shown to be NLRC4-dependent, but TLR5-independent [7].
Thus, it appears that TLR5 and NLRC4 are distinct sensors that respond to extracellular and cytosolic flagellin, respectively [8].
Further, it has been been demonstrated that NAIPs (NLRB subfamily members) serve as NLRC4 inflammasome receptors for flagellin and a conserved type III secretion system (TTSS) rod component [9].
NLRX1 (NOD9) is the first NLR protein shown to be localized at the mitochondria [10].
NLRX1 negatively impacts antiviral inflammatory response via the RIG-I/IPS-I sensing pathway [11, 12] and LPS-elicited responses via the TRAF6-IKK signaling pathway [12, 13].
Conversely, it has been shown that NLRX1 positively controls NF-κB and JNK signaling pathway to activate reactive oxygen species (ROS) production in response to TNF-α, poly(I:C), and pathogens [14, 15].
Further studies are needed to clarify the role of NLRX1 in antiviral immunity.
NLRPs (NALPs)
The NLRP subfamily consists of 14 members characterized by their PYD effector domains.
At least two types of NLRP inflammasomes have been identified: the NLRP1 inflammasome comprising NLRP1 (NALP1, CARD7), ASC, caspase-1 and caspase-5, and the NLRP3 inflammasome containing NLRP3 (NALP3, cryopyrin, CIAS1), ASC, Cardinal and caspase-1 [16].
NLRP1 and NLRP3 recruit through their PYD domain the adaptor protein ASC, which in turn interacts with caspase-1 via a CARD-CARD interaction. NLRP1 also recruits caspase-5 via its additional CARD effector domain at the C-terminus, whereas NLRP3, lacking such a CARD, interacts with the CARD-containing adaptor CARD8 (Cardinal) to recruit additional caspase-1.
Two molecules activate NLRP1: MDP and the anthrax toxin. NLRP1 oligomerization induced by MDP requires ATP. The anthrax toxin was shown to activate the murine variant of NLRP1 (NALP1b) suggesting NLRP1 inflammasome activation in the immune response to Bacillus anthracis infection [17].
NLRP3 mediates caspase-1 activation in response to a wide variety of stimuli: whole bacteria (L. monocytogenes, S. aureus), bacterial RNA, synthetic purine-like compounds (R848, R837), uric acid crystals, amyloid-b, extracellular ATP and pore-forming toxins (nigericin, maitotoxin) [18-20].
Mutations in NLRP3 are associated with inherited autoinflammatory diseases called cryopyrin-associated periodic syndromes (CAPS), such as familial cold autoinflammatory syndrome and Muckle-Wells syndrome [21].
References
1. Chamaillard M. et al., 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4: 702-707.
2. Girardin S. et al., 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300: 1584-1587.
3. Kobayash, K. et al., 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416: 194-199.
4. Kobayashi K. et al., 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731-734.
5. Hsu Y. et al., 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 8(2):198-205.
6. Ogura Y. et al., 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603-606.
7. Mariathasan S & Monack D., 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 7(1):31-40.
8. Zamboni D. et al., 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 7(3):318-25.
9. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477(7366):596-600.
10. Hong M. et al., 2012. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. the NALP3 inflammasome. Immunity. 36(3):337-47.
11. Moore C. et al., 2008. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451(7178):573-7. 12. Xia X. et al., 2011. NLRX1 negatively regulates TLR-induced NF-kB signaling by targeting TRAF6 and IKK. Immunity. 34(6):843-53.
13. Allen I. et al., 2011. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kB signaling pathways. Immunity. 34(6):854-65.
14. Abdul-Sater A. et al., 2010. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J Biol Chem. 285(53):41637-45.
15. Xiao T. & Ting J. 2012. NLRX1 Has a Tail to Tell. Immunity 36(3):311-2.
16. Martinon F. & Tschopp J., 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 117(5):561-74.
17. Boyden E. & Dietrich W., 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 38(2):240-4.
18. Kanneganti T. et al., 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440(7081):233-236.
19. Mariathasan S. et al., 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440(7081):228-32.
20. Sutterwala F. et al., 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 24(3):317-27.
21. Ting J. et al., 2006. CATERPILLERs, pyrin and hereditary immunological disorders. Nat. Rev. Immunol. 6: 183-195.