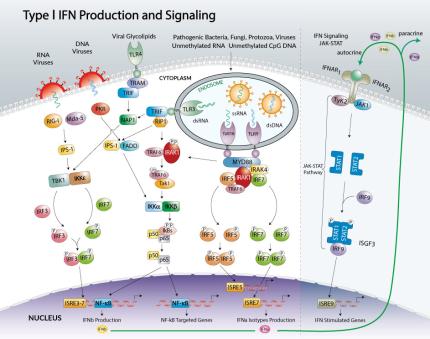
The most studied members of the Type I family of interferons are the multiple IFNα isotypes and IFNβ. Type I IFNs are responsible for inducing transcription of a large group of genes which play a role in host resistance to viral infections, as well as activating key components of the innate and adaptive immune systems including antigen presentation and production of cytokines involved in activation of T cells, B cells, and natural killer cells.
Type I IFNs are transcriptionally regulated, and are induced following recognition of pathogen components during infection by various host pattern recognition receptors. Virtually all humans cells are able to synthesize IFNα/β, however some cells have a more pronounced ability to produce these cytokines. Table 1 summarizes some characteristics of the three main pathways leading to the production of Type I IFN. The RIG-I pathway is activated upon infection by RNA viruses. The second pathway involves the adaptor protein TRIF which is recruited by TLR3 and TLR4. The last pathway is triggered by TLR7/8 and TLR9 leading to the activation of the transcription factor IRF7. Following their production, Type I IFNs trigger antiviral responses by binding to a common receptor (IFNAR). IFNα/β binding to IFNAR stimulates the JAK1-STAT pathway leading to the assembly of the ISGF3 complex which is composed of STAT1-STAT2 dimers and IRF9. ISGF3 binds to IFN-stimulated response elements (ISRE) in the promoters of IFN-stimulated genes to regulate their expression. Among these genes is IRF7 which initiates the transcription of a second wave of Type I IFNs. This autocrine/paracrine feed-back allows Type I IFNs to create an antiviral state in surrounding cells.
Table 1: Three major pathways involved in IFNα/β production in humans and mice.
| PATHWAYS | MAIN ACTORS | LOCALIZATION | INDUCERS | CELLS | REFERENCES |
| RIG-I pathway | RIG-I (MDA-5) | Cytoplasmic | Many single- and double-stranded RNA viruses | Conventional DCs, Fibroblasts, Hepatocytes | 1, 2, 3, 4, 5, 6 |
| TRIF pathway | TLR3-TRIF | Internal vesicles | Unmethylated dsRNA | Macrophages, Hepatocytes | 4, 5, 6, 7, 8 |
| TLR4-TRIF | Plasma membrane | Viral glycolipids | |||
| IRF7 pathway | TLR9-MyD88-IRF7(IRF5) | Endosome | Unmethylated RNA from pathogens and damaged host cells | Plasmacytoid DCs | 8, 9, 10 |
| TLR7/8-MyD88-IRF7(IRF5) | Unmethylated CpG DNA, Chromatin immuno complexes |
References:
1. Kato H. et al., 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 23(1):19-28.
2. Yoneyama M. et al., 2005. Shared and Unique Functions of the DExD/H-Box Helicases RIG-I, MDA5, and LGP2 in Antiviral Innate Immunity. J Immunol. 175(5):2851-8.
3. Kawai T. et al., 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated Type I interferon induction. Nat Immunol. [Epub ahead of print]
4. Li K. et al., 2005. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 280(17):16739-47.
5. Perry AK. et al., 2005. The host Type I interferon response to viral and bacterial infections. Cell Res 15:407.
6. Gale M. Jr, Foy EM., 2005. Evasion of intracellular host defence by hepatitis C virus. Nature. 436(7053):939-45.
7. Schroder M., Bowie AG., 2005. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 26(9):462-8.
8. Kariko K. et al., 2005. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity. 23(2):165-75.
9. Honda K. et al., 2005. IRF-7 is the master regulator of Type-I interferon-dependent immune responses. Nature. 434(7034):772-7.
10. Rifkin IR. et al., 2005. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 204:27-42. Review.