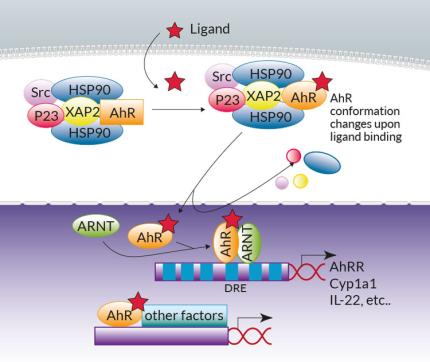
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcriptional factor widely expressed among immune, epithelial, endothelial and stromal cells in barrier tissues1. While historically studied in the context of chemical pollutants such as dioxin, AhR was more recently revealed as a central sensor of a wider range of environmental cues, ensuring intestinal homeostasis between the host and gut microbiota1.
AhR canonical signaling has been extensively reviewed1. Briefly, in the absence of ligands crossing the cell membrane, AhR resides in the cytoplasm within a Hsp90:XAP2:p23:Src chaperone protein complex. Upon ligand binding, the complex undergoes conformational changes and translocates into the nucleus. The chaperones are released and AhR heterodimerizes with AhR nuclear translocator (ARNT). The AhR:ligand:ARNT trimer binds to dioxin response elements (DREs) in the upstream regulatory regions of AhR target genes, which include the cytochrome P450-dependent monooxygenase Cyp1a1, the AhR repressor (AhRR), and the IL-22 interleukin. Of note, non-canonical AhR signaling pathways have also been reported, either at the genomic level through association with other transcription factors (e.g. NF-κB), or at the non-genomic level (e.g. through the release of the Src kinase)2,3.
Besides xenobiotics, including the prototypic AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a variety of dietary-derived AhR ligands have been identified, many of which are byproducts of tryptophan (Trp) amino acid metabolism4. AhR has emerged as a regulator of host-microbiome symbiosis. On the one hand, AhR activation by dietary ligands shapes the intestinal bacterial composition5. On the other hand, AhR sensing regulates homeostasis and functionality of the gut immune cells6.
Trp metabolism by the gut microbiota generates AhR agonists which support the development and maintenance of intestinal type 3 innate lymphoid cells (ILC3), the innate counterpart of the adaptive CD4 T cells producing IL-17 and IL-22 (Th17/22). AhR-signaling is also needed for the maintenance of IL-22 producing intraepithelial lymphocytes (IELs). IL-22 is involved in mucosal wound-healing and the production of anti-microbial peptides (AMPs) by intestinal epithelial cells (IECs)6. The AhR-IL-22 axis in the gut plays a significant role in the host defense against microbial pathogens, while simultaneously ensuring disease tolerance to limit harmful impact. To this end, there is increasing evidence that the strength of AhR activation modulates CD4 T cell responses. Weak AhR activation supports a pro-inflammatory response (Th17/22), while strong AhR activation promotes induction of tolerogenic DCs and regulatory T cells (Tregs)6-8.
Different sets of data may provide mechanistic explanations as to how AhR is involved in the regulation of both pro-inflammatory and tolerance responses. While canonical AhR signaling in intestinal immune cells leads to IL-22 production, non-genomic AhR signaling is responsible for at least two suppressive outcomes. Overreacting responses to LPS challenge in mice are repressed through AhR-associated Src kinase phosphorylation of the indoleamine 2,3-dioxygenase IDO1, which in turn induces NF-κB-mediated transcription of the suppressive cytokine TGF-β (transforming growth factor) in DCs9. Moreover, Trp degradation by IDO1 provides AhR agonists, such as L-Kynurenine, implicated in Treg generation9. Another LPS-induced suppressive effect is the production of the anti-inflammatory cytokine IL-10 via AhR-associated Src kinase and STAT3 signaling in macrophages. Of note, LPS recognition by TLR4 promotes AhR expression in macrophages, but it is unclear whether it is also the case upon stimulation with other pattern recognition receptor ligands10.
A series of diseases are associated with gut microbiota-immune cells dysbiosis, and whether imbalance is a cause or a consequence remains unclear. In inflammatory bowel disease, immune cells tend to express low levels and impaired activity of AhR, a status maintained by decreased concentration of gut microbiota-derived AhR ligands2,11. Colorectal cancer patients display alterations in gut microbiota, increased expression of AhR, chronic IDO1 activation, and reduced Trp concentration in the tumor micro-environment, supporting immune response suppression11. Contrarily, spondyloarthritis patients with gastrointestinal symptoms have low gut microbial diversity and Trp catabolism, consistent with inflammation11. Also, decreased circulating microbial catabolites of dietary Trp is implicated in pathogenesis of multiple sclerosis3. Hence, AhR plays a key role in gut-microbiota and host homeostasis, not only in the intestine but also at distant sites. Modulation of the AhR pathways is an attractive therapeutic strategy. However, it will require better understanding of AhR activation in different cell types, under varying doses and sources of ligands.
References
1. Stockinger B. et al., 2014. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32:403-32.
2. Lamas B. et al., 2018. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 11(4):1024-38.
3. Gutiérrez-Vasquez C. et al., 2018. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 48:19-33.
4. Hubbard T.D. et al., 2015. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 43:1522-35.
5. Murray I.A. et al. 2016. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci. Rep. 6:33969.
6. Cervantes-Barragan L. & Colonna M. 2018. AHR signaling in the development and function of intestinal immune cells and beyond. Semin. Immunopathol. 40(4):371-77.
7. Ehrlich A.K. et al. 2018. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol. Sci. 161(2):310-20.
8. Boule L.A. et al. 2018. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci. Rep. 8:1826.
9. Bessede A. et al. 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 511:184-90.
10. Zhu J. et al. 2018. Aryl hydrocarbon receptor promotes IL-10 expression in inflammatory macrophages through Src-STAT3 signaling pathway. Front. Immunol. 9:2033.
11. Gao J. et al. 2018. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 8:13.